Sodium fluorescein contrast agent modified by polyethyleneimine and its preparation method and application
A technology of polyethylenimine and fluorescein sodium, which is applied in preparations for in vivo experiments and pharmaceutical formulations, can solve problems such as patients’ inability to get a diagnosis early, delay in treatment timing, and adverse reactions of patients, so as to reduce tissue adsorption, The effect of maintaining the contrast effect and reducing the toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation and Characterization of PEI-NHAc-FS
[0036] 1 Preparation of PEI-NHAc-FS
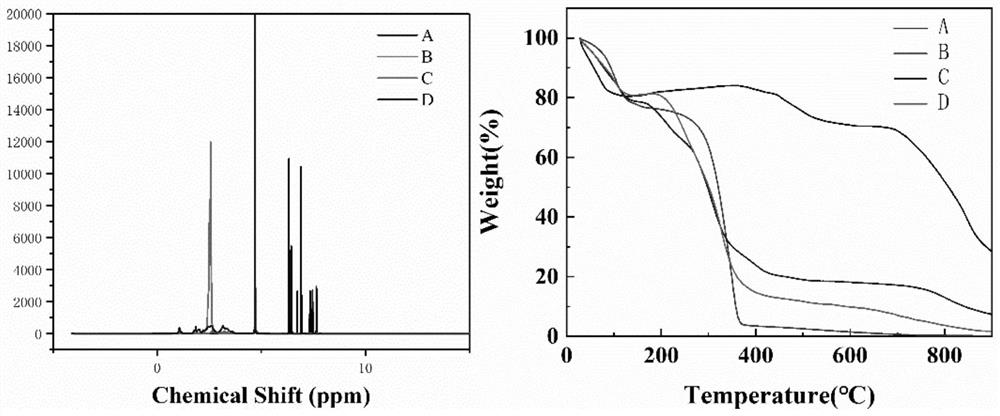
[0037] Sodium fluorescein (200 mg, 1 ml) was stirred with 10 molar equivalents of EDC·HCl for 30 minutes and then with 10 molar equivalents of NHS for an additional 3 hours to form an orange solution. Then, PEI (380 mg, 5 mL) with a molecular weight of 25,000 was added to the above solution and stirred vigorously for 3 days to obtain PEI-NH 2- FS. Afterwards, the PEI-NH 2- FS acetylation. Briefly, acetic anhydride (1 mL), which exceeded five times the molar equivalent of the remaining amino groups on PEI, was added to the above mixture and stirred for 24 hours, after which the mixture was thoroughly dialyzed for 3 days and freeze-dried to obtain PEI-NHAc-FS.
[0038] 2 Characterization of PEI-NHAc-FS
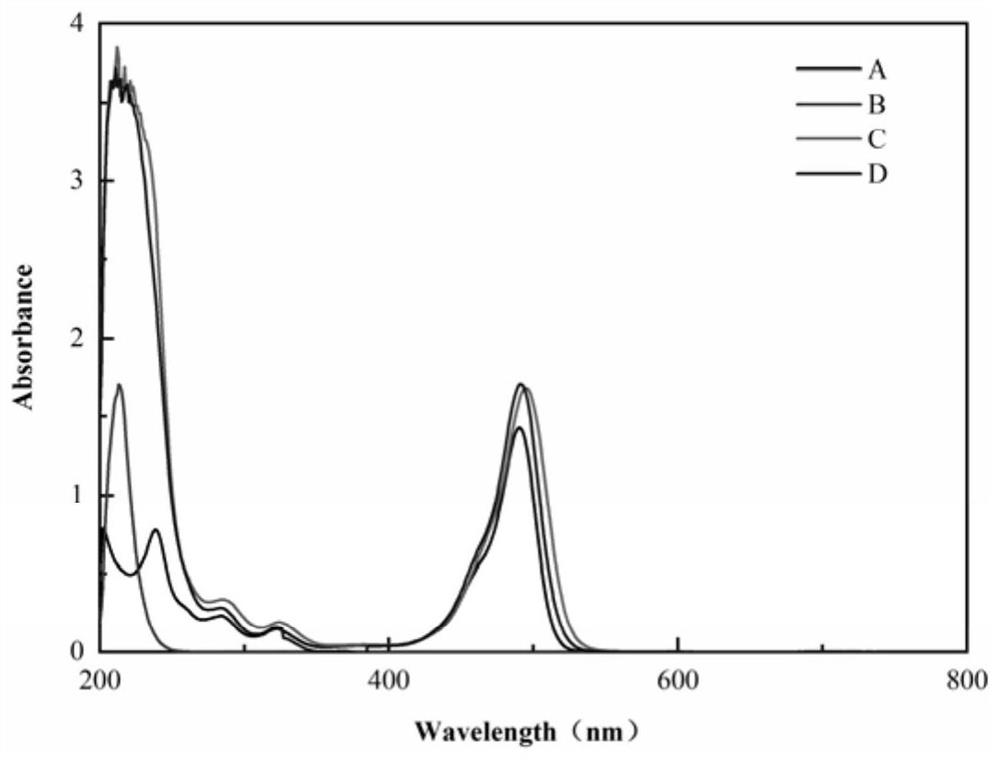
[0039] In order to observe the characteristics of the synthesized nanomaterials, FS, PEI, PEI-NH 2 -FS and PEI-NHAc-FS for characterization analysis, mainly including: ...
Embodiment 2
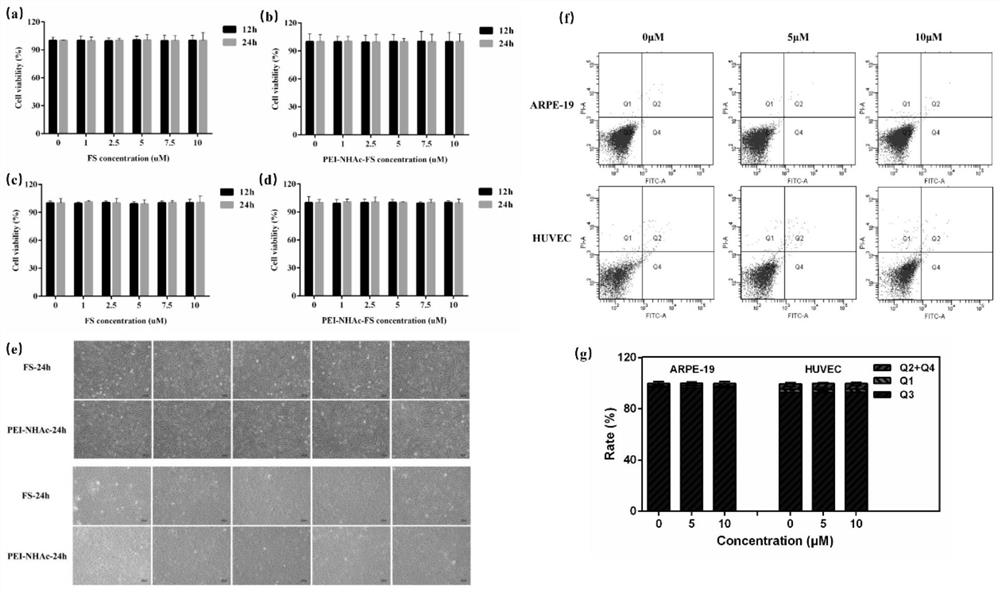
[0046] Example 2 Safety and effectiveness of PEI-NHAc-FS at the cellular level
[0047] 1 Experimental method
[0048] 1.1 CCK8 experiment
[0049] Take the ARPE-19 and HUVEC cells in the logarithmic growth phase, and take 10 4 Place cells / well into 96-well plates, put them in an incubator and cultivate them for 24 hours, and wait for the cells to adhere to the wall. Add 1-10μmol / L FS and PEI-NHAc-FS respectively, and incubate for 24h. Change the medium, add 110 μL of medium containing 10 μL of CCK-8 to each well, and incubate at 37°C for 2 hours in the dark. For detection on the machine, a wavelength of 450nm is selected, and the OD value of each well is detected on a multifunctional microplate reader.
[0050] 1.2 Detection of apoptosis by flow cytometry
[0051] Take the ARPE-19 and HUVEC cells in the logarithmic growth phase, and use 5×10 4 Each cell / well was spread into a 12-well plate, and cultured in an incubator for 24 hours until the cells adhered to the wall. ...
Embodiment 3
[0063] Example 3 Safety and effectiveness of PEI-NHAc-FS at animal level
[0064] 1 Experimental method
[0065] 1.1 Establishment of laser-induced CNV model in rats with BN
[0066] BN male rats (8-10 weeks, mass 180±20g), anesthetized by intraperitoneal injection of 1% pentobarbital sodium 40mg / mg, mydriasis with compound tropicamide, surface anesthetized with alcaine, exposed right eye , holding a cover glass as a contact lens, laser photocoagulation between 2 retinal vessels from the optic disc, a total of 6-8 points, using laser (532nm, 360mW, 0.1s, 50μm) to establish a rat CNV model, after photocoagulation Bubbles or mild bleeding shall prevail. Pay attention to avoid fundus blood vessels during laser treatment to avoid severe fundus hemorrhage.
[0067] 1.2 Preparation of paraffin sections
[0068]The above 6 BN rats were divided into 2 groups, control group (injection of normal saline into tail vein, n=3), PEI-NHAc-FS group (injection of 1% PEI-NHAc-FS 0.15mL in ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com