Cyclohexene catalytic oxidation cyclohexanone preparation reaction catalyst, and preparation method and application thereof
A technology for preparing cyclohexanone by catalytic oxidation of cyclohexene, which is used in the preparation of carbonyl compounds by oxidation, catalysts for physical/chemical processes, molecular sieve catalysts, etc. The effect of separation, high catalytic activity, excellent activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
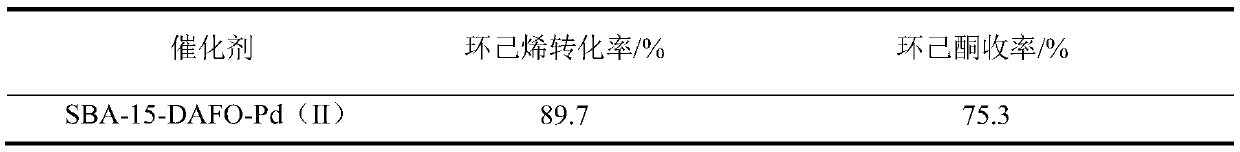
[0034] A method for preparing a catalyst for the catalytic oxidation of cyclohexene to cyclohexanone and a process for applying it to the reaction of catalytic oxidation of cyclohexene to cyclohexanone. The experimental steps are as follows:
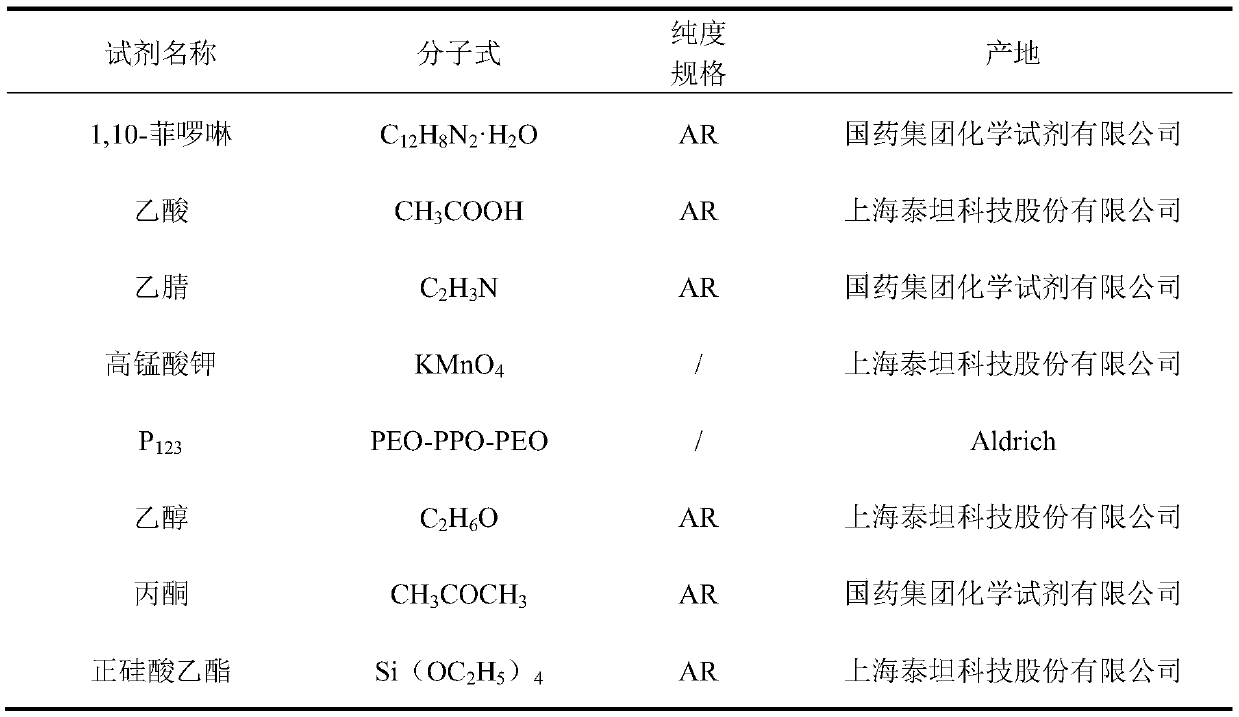
[0035] (1) Firstly, the synthesized mesoporous molecular sieve SBA-15 (synthesized according to the method reported in the literature) and aminopropyltriethoxysilane silicon coupling agent (the mass ratio of the two is 1:1~2) in the organic solvent toluene or bismuth Reflux reaction in toluene to prepare aminated SBA-15 material.
[0036] (2) Reflux the aminated SBA-15 material and 4,5-diazafluoren-9-one (DAFO) ligand (the mass ratio of the two is 1:1~2) in toluene or xylene for 12~ After 24 hours, the product was washed several times with absolute ethanol to obtain the modified SBA-15 carrier.
[0037] (3) After refluxing the modified SBA-15 carrier and PdCl2 (the mass ratio of the two is 30-100:1) in acetone for 36 hours, cooling and ...
Embodiment 1
[0046] Preparation and modification of SBA-15 mesoporous material:
[0047] (1) Preparation of SBA-15 molecular sieve material: 4g P123 (polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer) and 120g 2mol / L dilute in a 500ml three-necked flask Add hydrochloric acid and 30g of deionized water, and stir in a water bath at 38°C for 2 hours. After mixing evenly, add 8.4g of tetraethyl orthosilicate, continue stirring for 20h, and then move the solution to a Teflon-lined In an autoclave, put it in an oven at 100°C for 24 hours, cool it to room temperature, filter it, and wash it with deionized water and absolute ethanol several times, and dry the product in an oven for 6 hours to obtain a SBA-15 mesoporous material;
[0048] (2) Amination of SBA-15 mesoporous molecular sieves: under nitrogen protection, in a 250ml three-necked flask equipped with a condenser tube, add 2g of the prepared SBA-15 and 150ml of anhydrous xylene respectively, and stir at room temp...
Embodiment 2
[0050] Synthesis of 4,5-diazafluoren-9-one (DAFO) ligand
[0051] (1) Mix 3g of o-phenanthroline, 50ml of 1,4-dioxane solution, 2g of potassium hydroxide and 100ml of distilled water into a 500ml four-necked round bottom flask, and stir at a temperature of 100°C Reflux for 30 minutes to mix the solution evenly;
[0052] (2) 8g of KMnO 4 After mixing and dissolving with 60ml of water, add it dropwise into a four-neck flask through a constant pressure dropping funnel, complete the dropwise addition within half an hour, and then continue to reflux at 100°C for 1 hour;
[0053] (3) After the reflux was completed, it was filtered while it was hot to obtain a red solution, which was left standing in the refrigerator for 24 hours and filtered to obtain yellow needle-shaped crystals, and then recrystallized with ethanol to obtain the DAFO ligand.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com