Method for recovering high-purity products from acid washing waste liquid step by step
A high-purity technology for pickling waste liquid, applied in chemical instruments and methods, fluorosilicic acid, nitrogen compounds, etc. Sodium fluorosilicate is difficult to achieve and other problems, to achieve the effect of good recovery effect, low cost and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
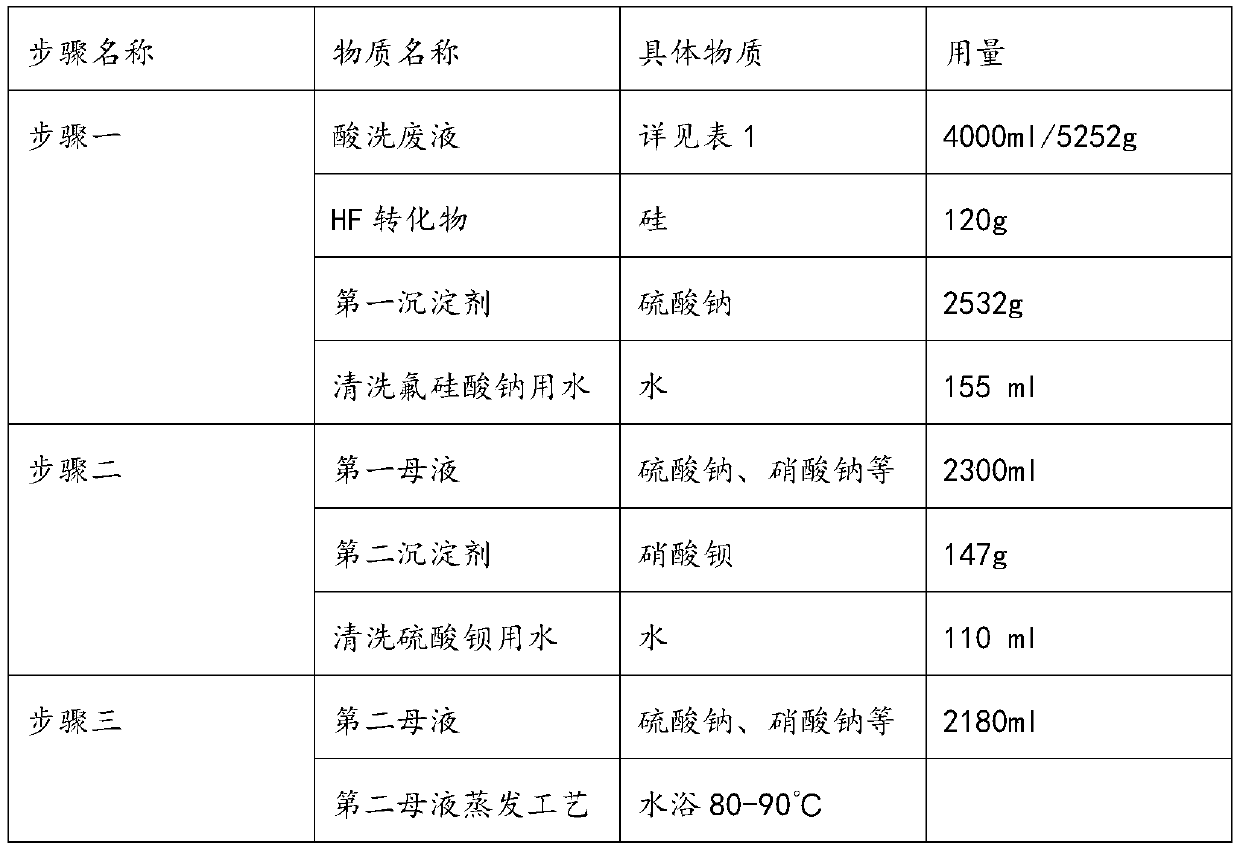
[0046] The composition and substance content of the pickling waste liquid used in this embodiment are shown in Table 1 for details.
[0047]Pickling waste liquor composition and content list in the embodiment 1 of table 1
[0048] component name Mass fraction of each substance (%) h 2 SiF 6
10.8 HF 5.2 HNO 3
6.9 h 2 SO 4
0.8 Na 0.8 h 2 o
75.5
[0049] A method for recovering high-purity products step by step from pickling waste liquid of the present embodiment comprises the following steps:
[0050] Step 1, reclaim fluorosilicate;
[0051] First add silicon to the pickling waste liquid to convert HF in the waste liquid into H 2 SiF 6 ;
[0052] and then converted to H 2 SiF 6 The first precipitating agent is added in the waste liquid, and the first precipitating agent is sodium sulfate.
[0053] Sodium fluorosilicate is generated, and the precipitate is filtered to obtain the first mother liquor;
...
Embodiment 2
[0077] The main difference between this embodiment and embodiment 1 is:
[0078] The HF conversion used in step one is SiO 2 , the first precipitant is sodium chloride; the second precipitant is barium chloride in step 2, and the specific consumption is shown in table 5.
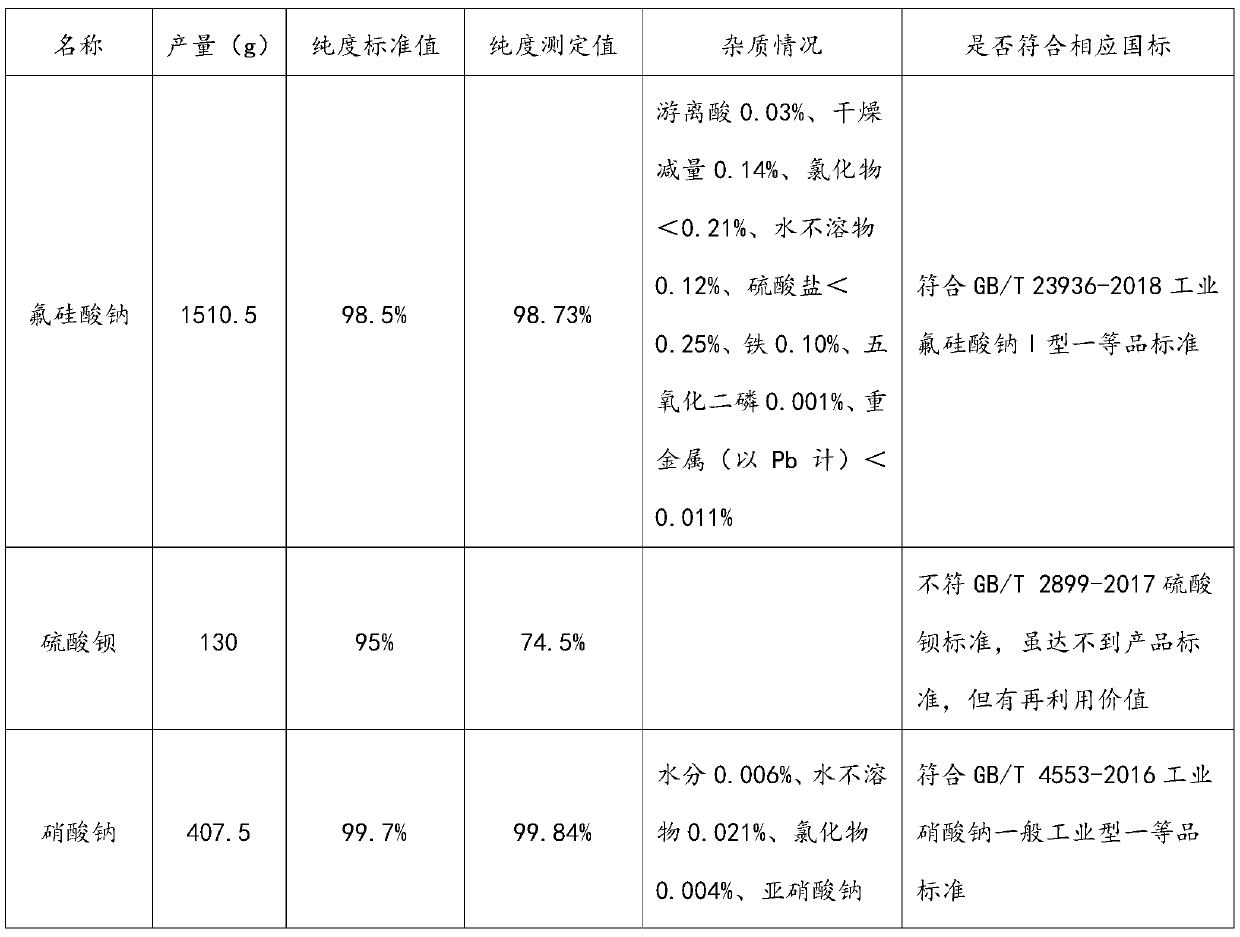
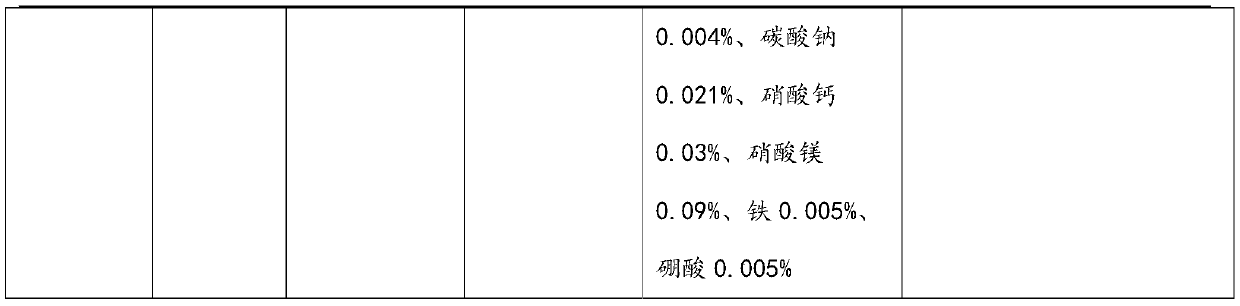
[0079] The output, purity and corresponding impurity situation of the target product sodium fluorosilicate, barium sulfate and sodium nitrate that the present embodiment reclaims are shown in Table 6 for details. See Table 7 for the composition of the solution after cooling and crystallization in Step 3.
[0080] Table 5 embodiment 2 step one to step three used process conditions, material and consumption situation
[0081]
[0082]
[0083] Table 6 embodiment 2 reclaims target product purity situation
[0084]
[0085] Solution composition situation after step 3 cooling crystallization in the embodiment 2 of table 7
[0086] main components quality score sodium sulfate 1.13% ...
Embodiment 3
[0088] The main difference between this embodiment and embodiment 1 is:
[0089] The HF conversion used in step one is SiO 2 , the first precipitant is sodium hydroxide; the second precipitant is barium chloride in step 2, and the specific consumption is shown in table 8.
[0090] The output, purity and corresponding impurity situation of the target product sodium fluorosilicate, barium sulfate and sodium nitrate that the present embodiment reclaims are shown in Table 9 for details. The composition of the solution after cooling and crystallization in step 3 is shown in Table 10.
[0091] Table 8 embodiment 3 steps one to step three used process conditions, material and consumption situation
[0092]
[0093] Table 9 embodiment 3 reclaims target product purity situation
[0094]
[0095]
[0096] The composition of the solution after step 3 cooling and crystallization in Table 10 Example 3
[0097] main components quality score sodium sulfate 1.5%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com