Iridium metal complex and preparation method and application thereof
An iridium metal complex, selected technology, applied in the direction of indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., can solve the problems of high synthesis price of phosphorescent materials, high synthesis process requirements, easy pollution of the environment, etc., to achieve Effects of improved luminous efficiency, low driving voltage, high luminous efficiency and brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The present invention also provides a kind of preparation method of above-mentioned iridium metal complex, comprises the following steps:
[0067] Under the condition of shielding gas, raw material a and raw material b are reacted in the first solvent, obtain the iridium metal complex with the structure shown in formula (I);
[0068] The raw material a has a structure shown in formula (II-1), the raw material b has a structure shown in formula (III-1); or the raw material a has a structure shown in formula (II-2), the The raw material b has a structure shown in formula (III-1) or has a structure shown in formula (III-2);
[0069]
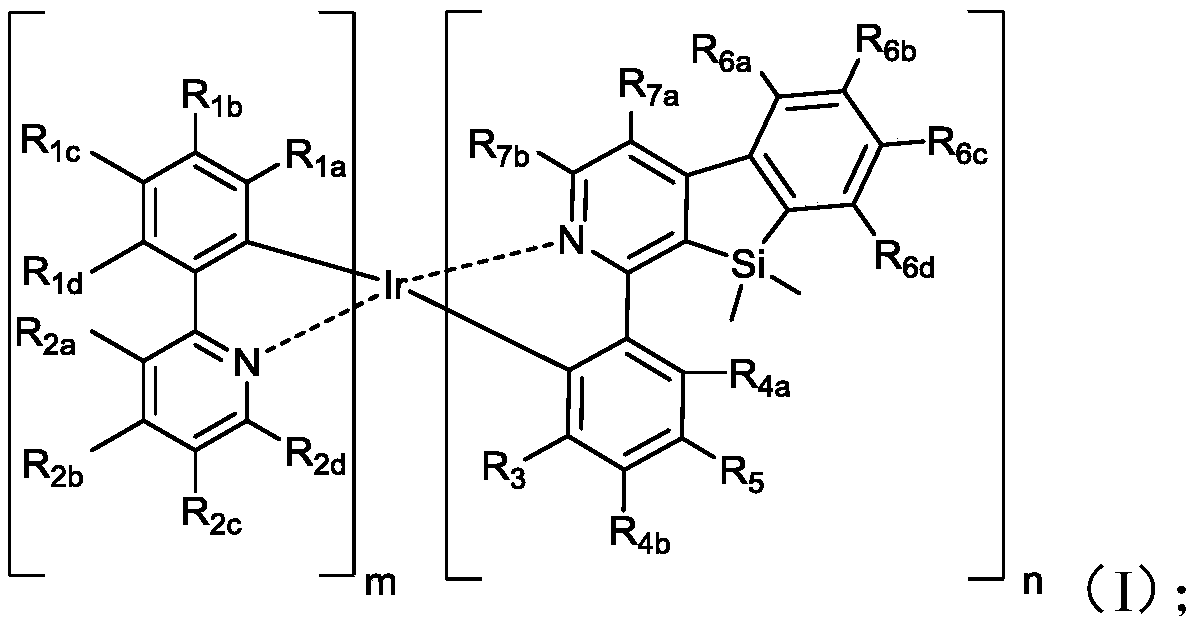
[0070] Wherein, m is an integer of 0 to 2, n is an integer of 1 to 3, and m+n=3;
[0071] R 1a , R 1b , R 1c , R 1d , R 2a , R 2b , R 2c , R 2d , R 3 , R 4a , R 4b , R 5 , R 6a , R 6b , R 6c , R 6d , R 7a and R 7b independently selected from hydrogen, deuterium, nitro, amino, hydroxyl, halogen, cyano, mercapto, alkyl, al...
Embodiment 1
[0123] Raw material d has the structure shown in formula (V-1), wherein, R 1a , R 1b , R 1c , R 1d , R 2a , R 2b , R 2c , R 2d Both are -H;
[0124] Raw material c has a structure shown in formula (IV-1), wherein, R 1a , R 1b , R 1c , R 1d , R 2a , R 2b , R 2c , R 2d Both are -H;
[0125] Raw material a has a structure shown in formula (II-1), wherein, R 1a , R 1b , R 1c , R 1d , R 2a , R 2b , R 2c , R 2d Both are -H;
[0126] Raw material b has the structure shown in formula (Ⅲ-1), wherein, R 3 , R 4a , R 4b , R 5 , R 6a , R 6b , R 6c , R 6d , R 7a and R 7b Both are -H;
[0127] Step 1. Under the nitrogen protection system, weigh raw material d (64.5mmol, 10.01g), IrCl 3 ·H 2 O (24.8mmol, 8.75g) was put into the reaction system, a mixed solution of 300mL ethylene glycol ether and 100mL pure water was added, refluxed at 120°C for 24h under the protection of nitrogen, and then cooled to room temperature, a precipitate was precipitated, and th...
Embodiment 2
[0136] Raw material d, raw material c, raw material a are all identical with embodiment 1;
[0137] Raw material b has the structure shown in formula (Ⅲ-1), wherein, R 4a , R 6a , R 6c , R 6d , R 7a and R 7b Both are -H; R 6b , R 3 , R 5 and R 4b for -CH 3 ;
[0138] Step 1, step 2 are all identical with embodiment 1;
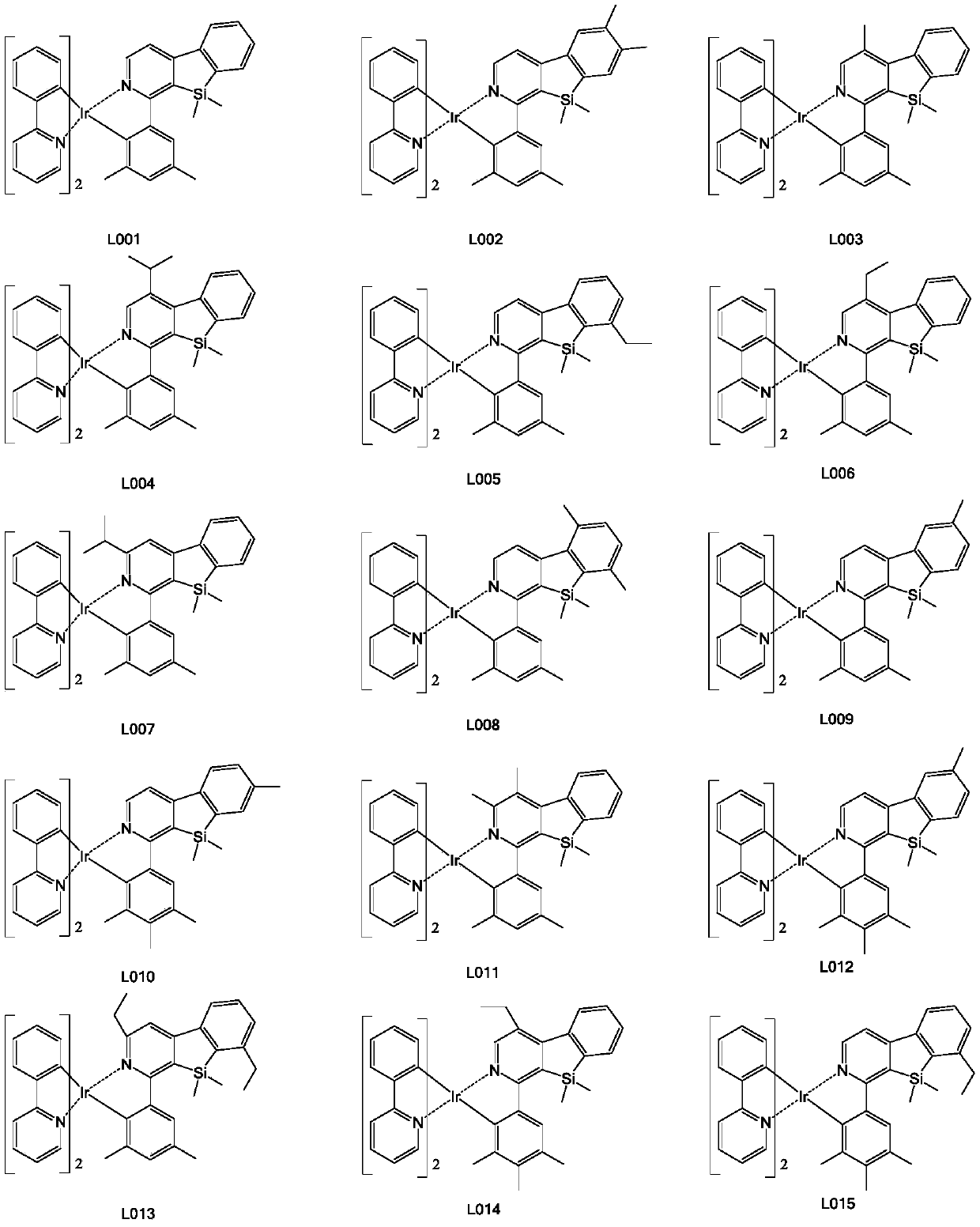
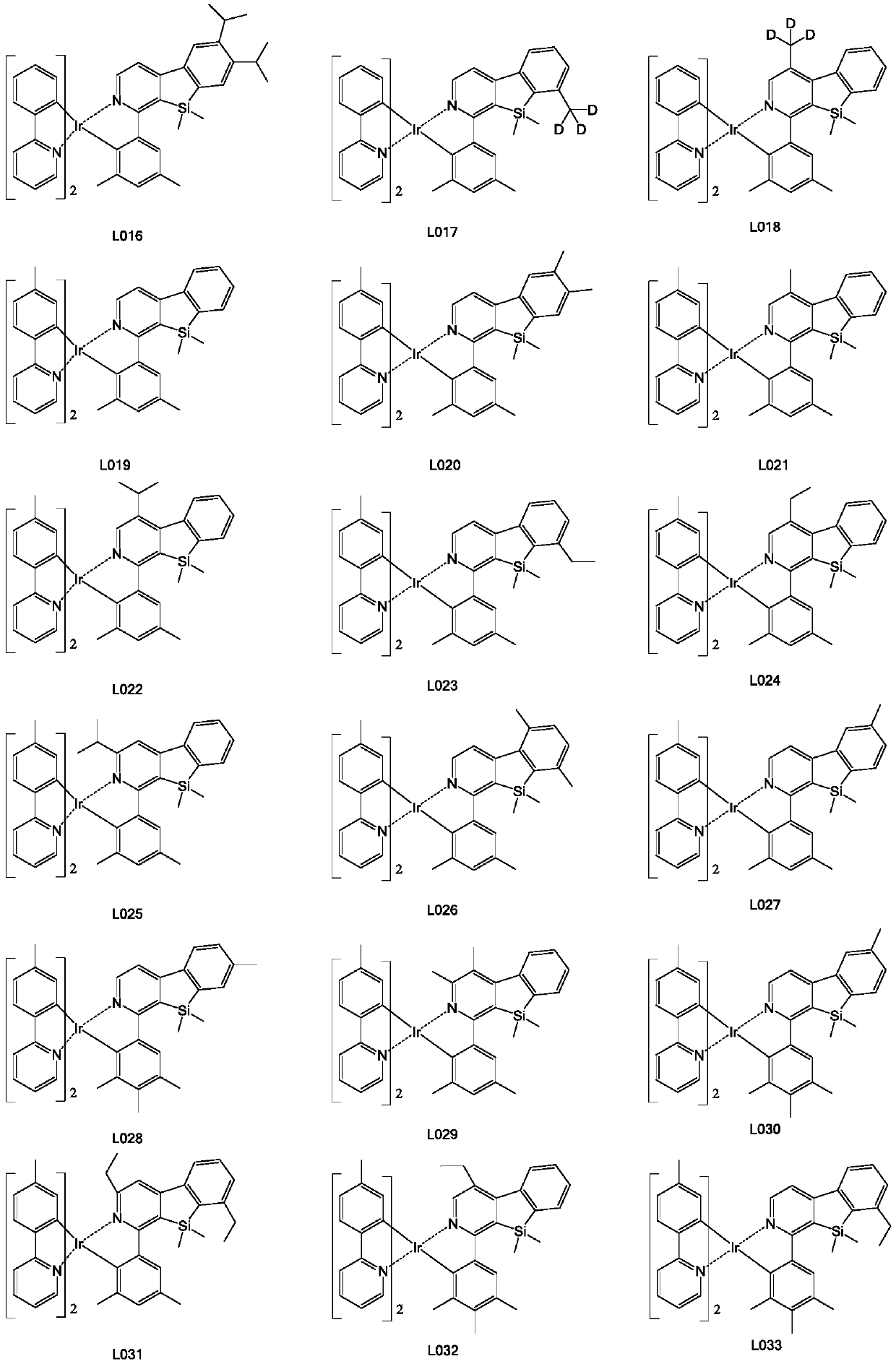
[0139] Step 3. Weigh raw material a (8.4mmol, 5.98g), add raw material b (25.5mmol, 8.76g), then add 120mL of absolute ethanol to the system, under the protection of nitrogen, reflux at 80°C for 24h, filter with suction, wash with alcohol , dry, use dichloromethane as solvent, use silica gel column chromatography, the filtrate is concentrated to solid precipitation, obtains the final yellow iridium metal complex (2.19g, yield 31%), has the structure shown in formula (L012) .
[0140] HPLC purity: 99.2%.
[0141] Mass Spectrum: Calculated 843.14; Found 844.25.
[0142] Elemental analysis:
[0143] Calculated values: C: 64.11%; H: 4.78%; N: 4.98%;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com