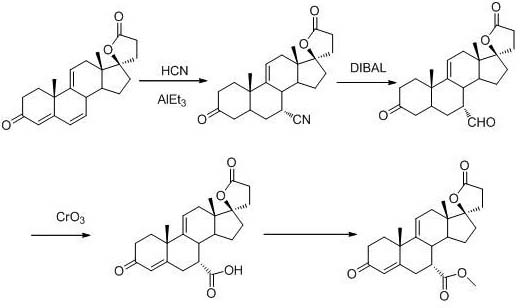
A method for synthesizing 7a-methyl formate-9(11)-encanrenone
A technology of encanrenone and methyl formate, which is applied in the field of medicine and chemical industry, can solve the problems of high toxicity and unsuitability for industrial production, achieve the effects of less pollution of three wastes, simplify redox re-oxidation and esterification operations, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
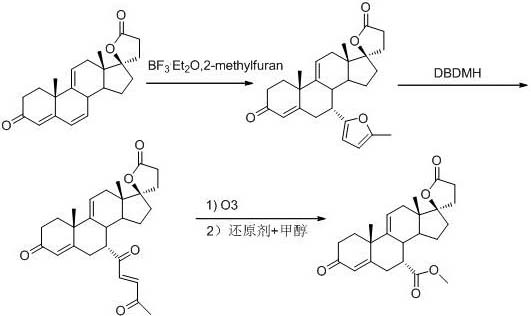
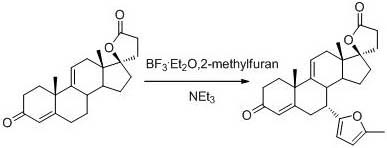
[0025] Add 20 g (0.0591 mol, 1.0 equiv) of 9(11)-encanrenone, 100 mL of acetonitrile, and 9.12 g (0.111 mol, 1.88 equiv) of 2-methylfuran into the reactor under nitrogen protection, and cool down to -20 ~-25°C, add 12.16g (0.0857 mol, 0.45 equiv) of boron trifluoride diethyl ether solution dropwise, after dropping, keep warm for 4 hours, TLC detection to control the reaction end point, after the reaction is completed, add 10 mL of triethylamine (0.0719 mol, 1.22 equiv) to stop the reaction, concentrate to dryness under reduced pressure, add 200 mL of dichloromethane and 100 mL of water, wash and separate layers, concentrate the organic layer to dryness under reduced pressure, recrystallize with methyl tert-butyl ether, filter, and dry to obtain white The solid 7a-(5-methyl-2furan)-9(11)-encanrenone was 18.2 g, the molar yield was 73.23%, and the HPLC purity was 98.92%.
[0026]
[0027] Add 20g (0.0476 mol, 1.0 equiv) of 7a-(5-methyl-2-furan)-9(11)-encanrenone, ...
Embodiment 2
[0032]
[0033] Add 20g of 7a-(1,4-dicarbonylpentene)-9(11)-encanrenone (0.0458mol, 1.0equiv), 200mL of methanol, and 200ml of dichloromethane to the reactor under nitrogen protection, and cool down To about -50°C, react with ozone, TLC to determine the end point of the reaction, the reaction is complete, replace with nitrogen, heat up to 0°C, add 30g of iron powder for reduction, test the non-oxidative properties of starch potassium iodide test paper, filter, and concentrate the filtrate under reduced pressure until a large amount of solids precipitate , put on a silica gel column, collect the chromatographic solution and concentrate to dryness to obtain 7.2 g of the target product, with a molar yield of 39.45% and an HPLC purity of 98.56%.
Embodiment 3
[0035]
[0036] Add 20g of 7a-(1,4-dicarbonylpentene)-9(11)-encanrenone (0.0458mol, 1.0equiv), 200mL of methanol, 200ml of toluene to the reaction kettle under the protection of nitrogen, and cool down to - At about 50°C, react with ozone, TLC determines the reaction end point, the reaction is complete, replace with nitrogen, keep warm to -50°C, add 2g of magnesium powder for reduction, test the non-oxidative properties of starch potassium iodide test paper, filter, and concentrate the filtrate under reduced pressure until a large amount of solids are precipitated. Put on a silica gel column, collect the chromatographic solution and concentrate to dryness to obtain 6.8 g of the target product, with a molar yield of 37.25% and an HPLC purity of 98.44%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com