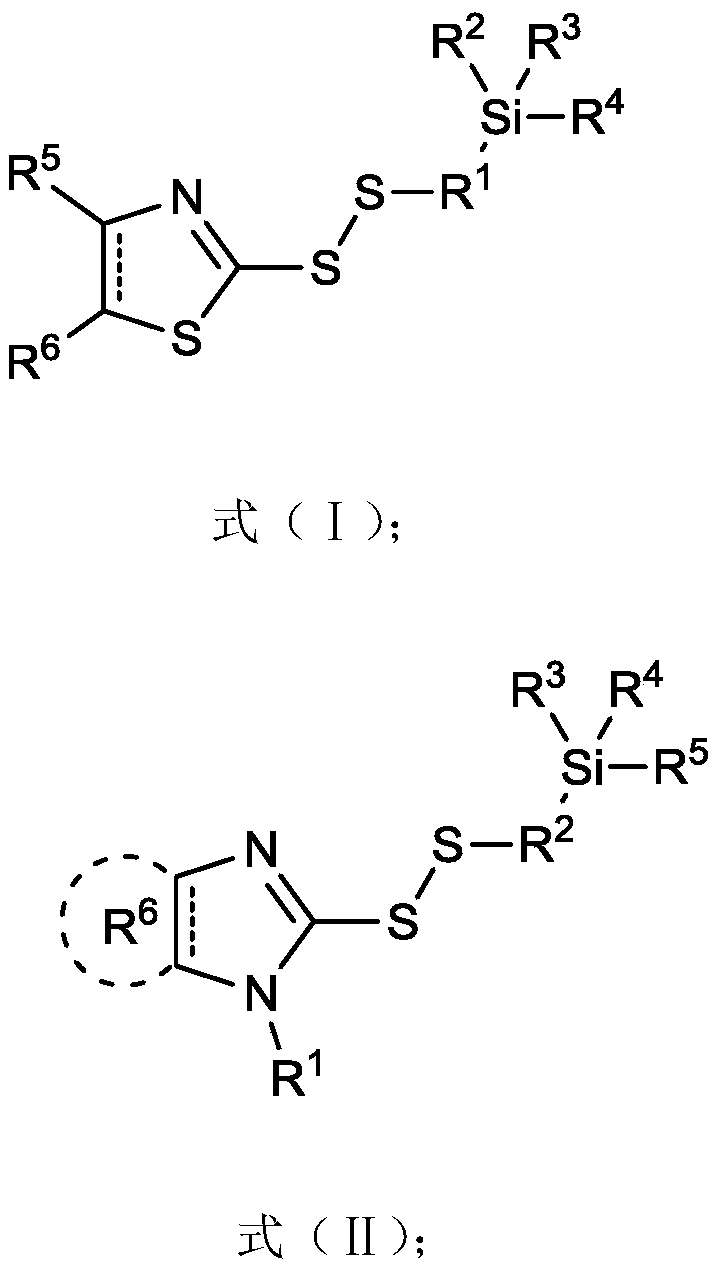
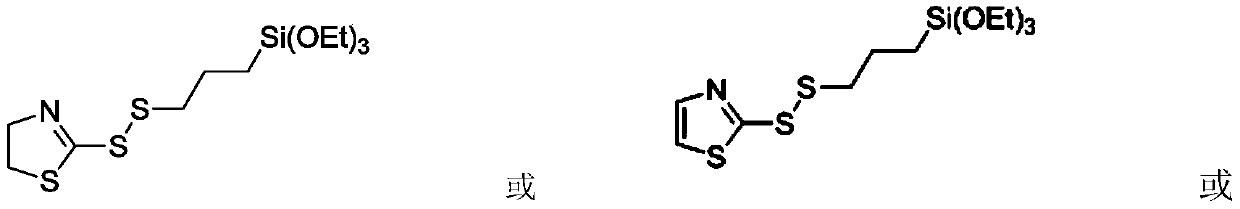
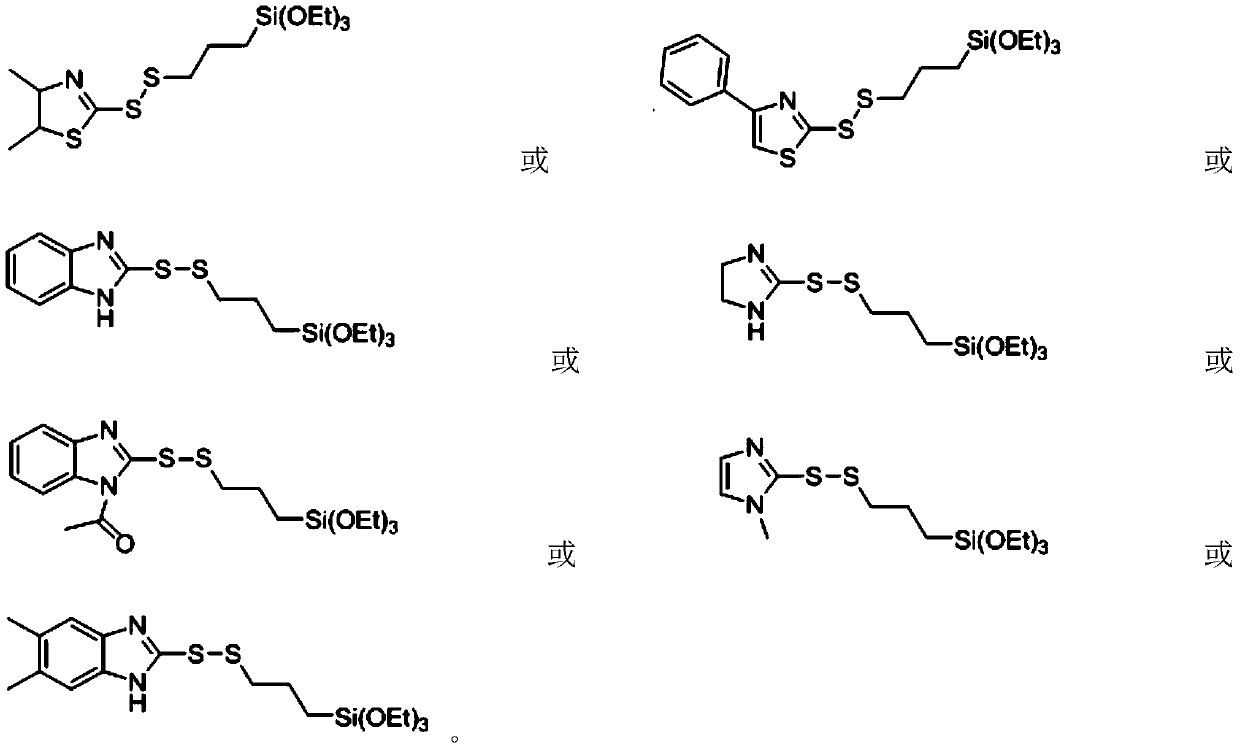
2-thiothiazolyl or 2-thioimidazolyl terminated mercaptosilane coupling agent as well as synthesis method and application thereof
A mercapto-terminated silane coupling agent and thioimidazoline group-capped technology, which is applied in the field of silane coupling agents, can solve the problem that silane coupling agents cannot improve the interaction between polymers and fillers, and shorten the vulcanization time , Promote vulcanization, reduce fuel consumption and carbon dioxide emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Under an inert gas nitrogen atmosphere, dissolve 23.84g (0.2mol) 2-mercaptothiazoline in 400mL toluene in a 1LSchlenk bottle to form a solution with a concentration of 0.5mol / L, and add 41.70mL (0.3mol) triethyl amine, then place the solution in an ice-water bath (0°C), add 65.50g (0.24mol) triethoxysilylpropylsulfenyl chloride dropwise to the solution system, and place it at room temperature after the dropwise addition, with a molar ratio of 1.2 : 1, the magneton rotating speed is 800r / min, and the reaction time is 12h, and TLC detects and tracks the reaction process. After the reaction was completed, the hydrochloride of triethylamine was removed by filtration, and the solvent was removed by a rotary evaporator, followed by column chromatography separation with 400 mesh silica gel powder, the eluent system was petroleum ether and ethyl acetate, and the gradient elution polarity The selection range was 8:1~5:1, and 48g of the new compound was obtained. The target comp...
Embodiment 2
[0062] Under an inert gas argon atmosphere, in a 500mL Schlenk bottle, 11.92g (0.1mol) 2-mercaptothiazoline was dissolved in 250mL carbon tetrachloride to form a solution with a concentration of 0.4mol / L, and 20.85mL (0.15 mol) triethylamine, then place the solution in an ice-water bath (0°C), add 30.02g (0.11mol) triethoxysilylpropylsulfenyl chloride dropwise into the solution system, place it at room temperature after the dropwise addition, The molar ratio was 1.1:1, the magneton rotational speed was 500r / min, the reaction time was 10h, and the reaction progress was tracked by TLC detection. After the reaction was completed, the hydrochloride of triethylamine was removed by filtration, and the solvent was removed by a rotary evaporator, followed by column chromatography separation with 400 mesh silica gel powder, the eluent system was petroleum ether and ethyl acetate, and the gradient elution polarity The selection range is 8:1~5:1, and compound 23g is obtained. Characteri...
Embodiment 3
[0065] Under an inert gas nitrogen atmosphere, dissolve 11.72g (0.1mol) of 2-mercaptothiazole in 150mL of dichloromethane in a 250mL Schlenk bottle to form a solution with a concentration of 0.67mol / L, and add 20.85mL (0.15mol) of three Ethylamine, then place the solution in an ice-water bath (0°C), add 32.75g (0.12mol) triethoxysilylpropylsulfenyl chloride dropwise into the solution system, place it at room temperature after the dropwise addition, and the molar ratio is 1.2:1, the magneton rotation speed is 600r / min, the reaction time is 10h, and the reaction process is tracked by TLC detection. After the reaction was completed, the hydrochloride of triethylamine was removed by filtration, and the solvent was removed by a rotary evaporator, followed by column chromatography separation with 400 mesh silica gel powder, the eluent system was petroleum ether and ethyl acetate, and the gradient elution polarity The selection range was 10:1~5:1, and 21g of the new compound was obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com