Nitroreductase fluorescent probe based on nitro reduction and sulfur-nitrogen transposition, and preparation method thereof
A technology of nitroreductase and fluorescent probe, which is applied in the field of chemical analysis to achieve the effects of low biological toxicity, specific fluorescence imaging detection, sensitive and selective detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthetic probe (1), its structure is:
[0045]
[0046] The synthesis technique of probe (1) is:
[0047]
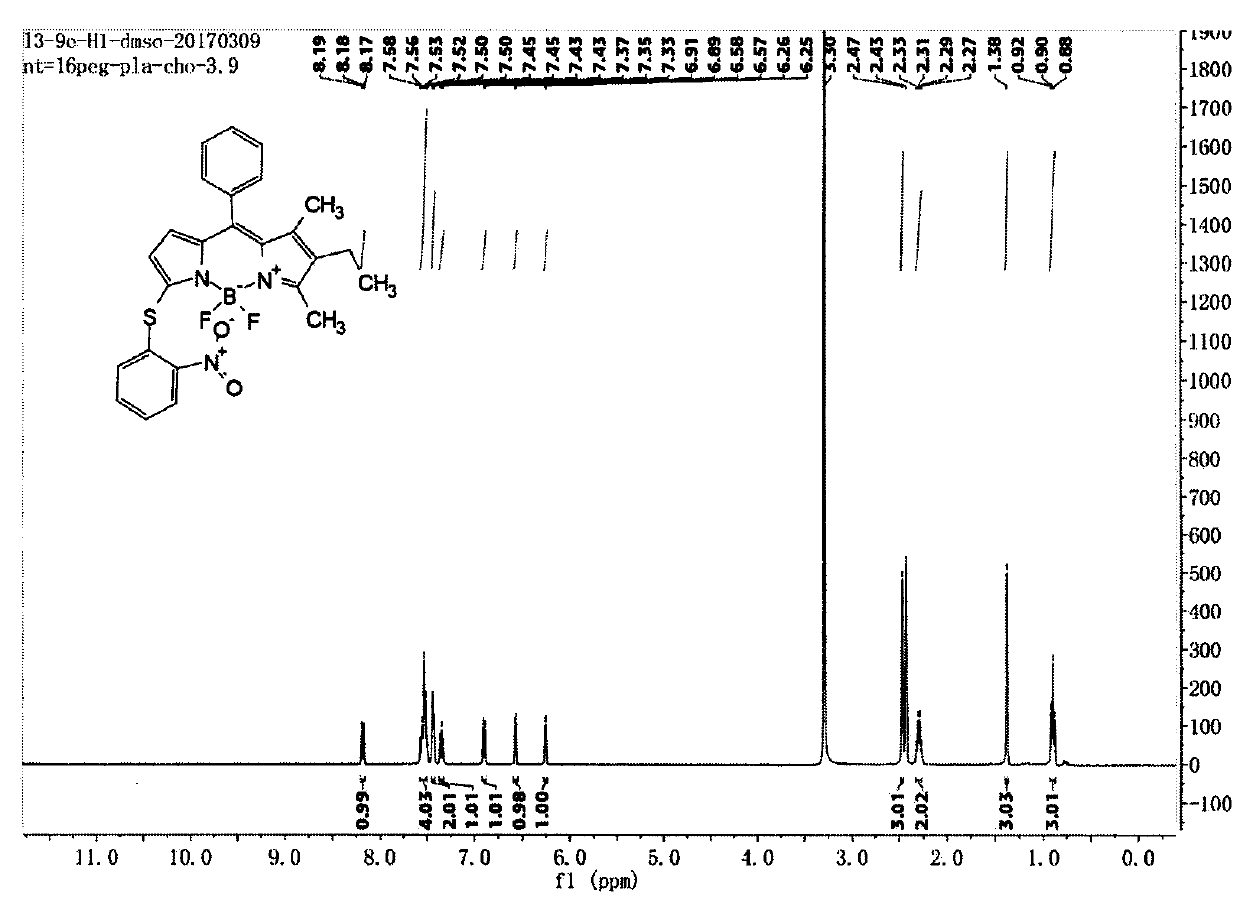
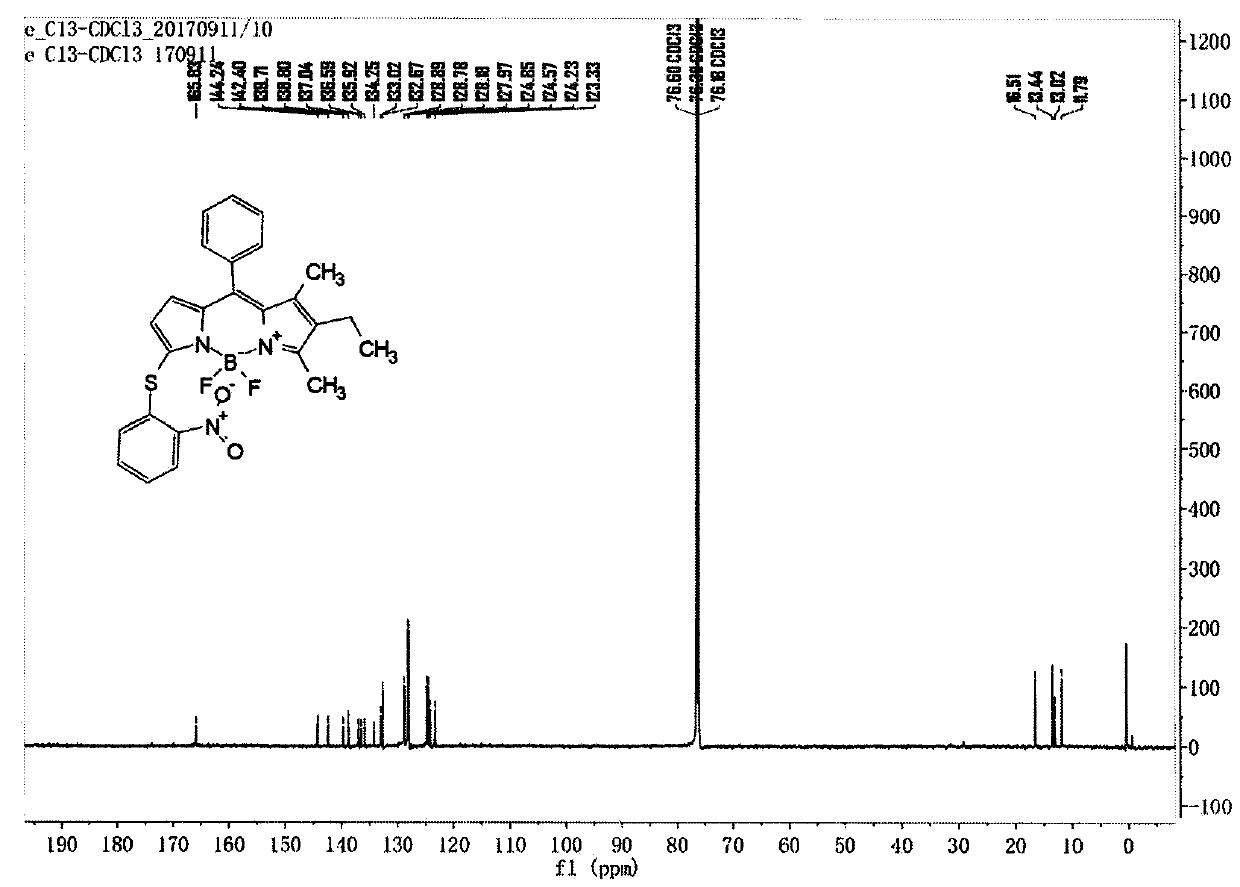
[0048] Compound C (122mg, 0.338mmol) and Compound e (105mg, 0.667mmol) were dissolved in dry dichloromethane, DMAP (124mg, 1.016mmol) was added, stirred at room temperature for 5 hours under nitrogen protection, the reaction solution was concentrated and passed through a silica gel column Chromatography (petroleum ether: ethyl acetate: dichloromethane = 20:1:1) gave compound (1), 62 mg, yield 38.5%. LC-MS (ESI) m / z: 477.1 [M+H] + ; 1 H NMR (400MHz, DMSO-d 6 )δ8.21-8.15(m, 1H), 7.60-7.48(m, 4H), 7.46-7.42(m, 2H), 7.35(t, J=7.8Hz, 1H), 6.90(d, J=8.2Hz , 1H), 6.57(d, J=3.9Hz, 1H), 6.26(d, J=3.8Hz, 1H), 2.47(s, 3H), 2.30(q, J=7.6Hz, 2H), 1.38(s , 3H), 0.90(t, J=7.5Hz, 3H); 13 CNMR(151MHz,Chloroform-d)δ165.83,144.24,142.40,139.71,138.80,137.04,136.59,135.92,133.02,132.67,128.89,128.78,128.18,127.97,124.85,124.57,124.23,123.33,16.51,13.44, 13.02, 11.79.
Embodiment 2
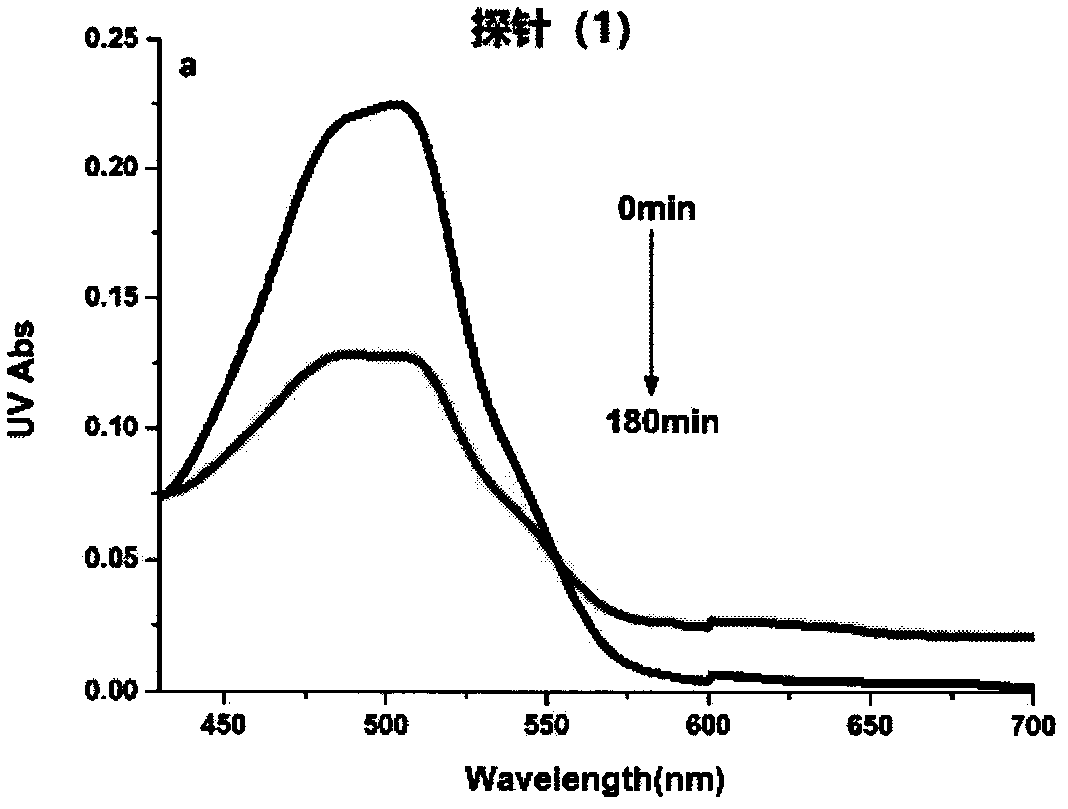
[0049] Example 2. Changes in UV absorption spectra before and after the reaction of probe (1) with nitroreductase
[0050] NADH is formulated into 0.15M Tris solution, which is ready-to-use; the probe is formulated into 3mM acetonitrile solution, which is used for subsequent use; nitroreductase (1mg, 90%) is prepared into a 3g / L stock solution with deionized water , aliquoted and stored at -20°C for later use. Add 3 mL of Tris (pH 7.4, 50 mM) buffer solution containing 25% acetonitrile into a 3 mL quartz cuvette, then add 10 μL of probe, 10 μL of NADH and 5 μL of nitroreductase in sequence. React at 37°C for 180min, and measure its absorption spectrum. Depend on image 3 It can be seen that the maximum ultraviolet absorption intensity at 505 nm of the probe (1) becomes weaker before and after the reaction.
Embodiment 3
[0051] Embodiment 3. Fluorescent emission spectrum changes before and after the probe (1) reacts with nitroreductase
[0052] Add 3 mL of Tris (50 mM, pH 7.4) buffer solution containing 25% acetonitrile to a 3 mL quartz cuvette, then add 10 μL of probe, 10 μL of NADH and 5 μL of nitroreductase (NTR) in sequence. React at 37°C for 180 min, and measure its fluorescence emission spectrum. The excitation wavelength is 470nm, the excitation slit width is 10nm, the emission slit width is 10nm, and the gain is 700V. Depend on Figure 4 It can be seen that the fluorescence of the probe (1) itself is weak, and the maximum fluorescence emission is 560 nm after reacting with nitroreductase, and the fluorescence is enhanced by about 7 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com