Catalyst, preparation method thereof, and application of catalyst in preparation of beta-isophorone
A technology of isophorone and catalyst, which is applied in the field of isophorone preparation technology, can solve the problems of equipment corrosion by strong alkaline catalyst, low purity of β-isophorone, poor dispersibility of β-cyclodextrin, etc. Achieve the effect of improving reaction selectivity, using less catalyst, and increasing base value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
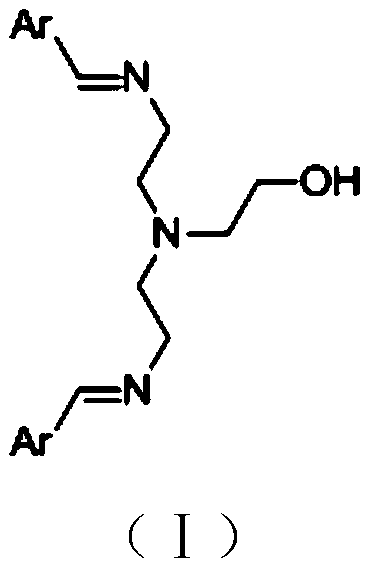
[0044] Preparation of N-(2-hydroxyethyl)diethylenetriamine-benzaldehyde Schiff base
[0045]
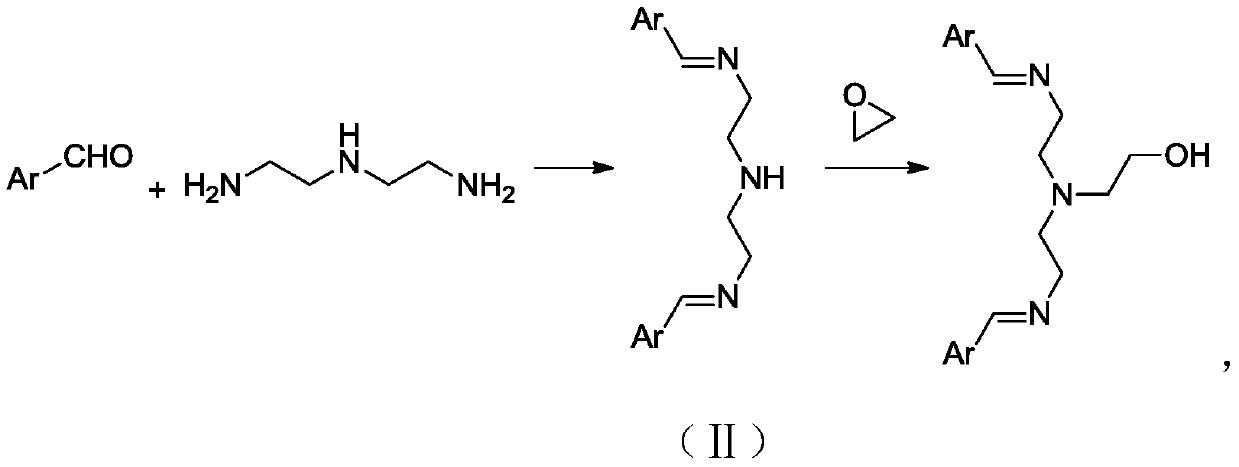
[0046] Add 10.3g (0.1mol) diethylenetriamine, 21.2g (0.2mol) benzaldehyde, and 500g ethanol to a 1L reaction flask in sequence, stir at 50°C for 6h, add 4.4g (0.1mol) ethylene oxide, and Stirring was continued for 2 hours, the reaction solution was directly filtered, and dried in a vacuum oven (60°C, 2KPa) to obtain 35.4g of N-(2-hydroxyethyl)diethylenetriamine-benzaldehyde Schiff base catalyst, which was designated as Catalyst A.
[0047] Product HPLC purity is 99.5%, melting point is 167.9-168.6 ℃,
[0048] HNMR (DMSO, 400M): = 8.78 (S, 2H, ArH), 7.85 (d, 4H, ArH), 7.61 (dd, 2H, ArH), 7.50 (m, 4H, ArH), 3.80 (S, 1H, -OH), 3.56-3.66 (m, 6H), 2.2-2.5 (m, 6H).
[0049] Add α-isophorone containing 0.005wt% catalyst A to the tower still of a plate tower reactor with 20 plates, and carry out refining under the conditions of 180°C, absolute pressure 0.05MPa, and reflux ratio of 30:1. ...
Embodiment 2
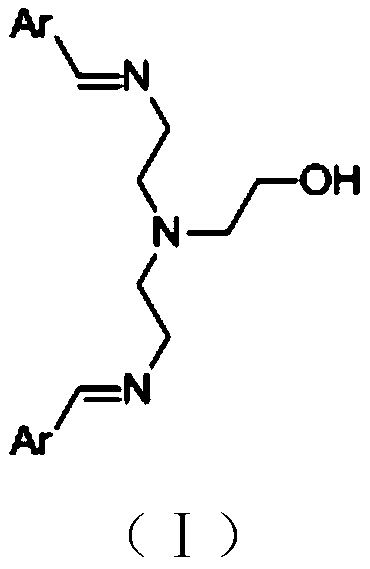
[0052] Preparation of N-(2-hydroxyethyl)diethylenetriamine-o-hydroxybenzaldehyde Schiff base
[0053]
[0054] Add 10.3g (0.1mol) diethylenetriamine, 30.5g (0.25mol) o-hydroxybenzaldehyde, 500g ethanol to a 1L reaction flask in sequence, stir at 70°C for 5h, add 3.96g (0.09mol) ethylene oxide, 20 Stirring was continued for 10 h at °C, the reaction solution was directly filtered, and dried in a vacuum oven (60 °C, 2KPa) to obtain 37.4 g of catalyst, which was designated as Catalyst B.
[0055] Product HPLC purity is 99.5%, melting point is 147.1-148.2 ℃,
[0056] HNMR (DMSO, 400M): = 8.68 (S, 2H, ArH), 7.21-7.65 (m, 8H, ArH), 5.81 (s, 2H, Ar-OH), 3.76 (S, 1H, -OH), 3.52 -3.61 (m, 6H), 2.21-2.50 (m, 6H).
[0057] Add α-isophorone containing 0.01wt% catalyst B to the tower still of a plate tower reactor with 25 plates, and carry out refining under the conditions of 230°C, absolute pressure 0.1MPa, and reflux ratio of 30:1. Distillation reaction, isomerization reaction occur...
Embodiment 3
[0059] Preparation of N-(2-hydroxyethyl)diethylenetriamine-furfuraldehyde Schiff base
[0060]
[0061] Add 10.3g (0.1mol) diethylenetriamine, 15.4g (0.16mol) furfuraldehyde, 500g ethanol to a 1L reaction flask in sequence, stir at 20°C for 10h, add 4.8g (0.11mol) ethylene oxide, and Continue to stir for 0.5h, filter the reaction solution directly, and dry it in a vacuum oven (60°C, 2KPa) to obtain 30.2g of N-(2-hydroxyethyl)diethylenetriamine-furanformaldehyde Schiff base catalyst, denoted as Catalyst C .
[0062] Product HPLC purity is 99.8%, melting point is 133.6-134.2 ℃,
[0063] HNMR (DMSO, 400M): = 7.68 (d, 2H, ArH), 7.38 (d, 2H, CH), 6.51-6.68 (m, 4H, ArH), 3.56 (S, 1H, -OH), 3.42-3.51 (m, 8H), 1.6-1.69 (m, 4H).
[0064] Add α-isophorone containing 1wt% catalyst A to the still of a plate tower reactor with 30 plates, and carry out rectification at 280°C, absolute pressure 0.2MPa, and reflux ratio of 3:1 reaction, an isomerization reaction occurs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com