Temozolomide prodrug nano-micelles and preparation method therefor and application of temozolomide prodrug nano-micelles
A temozolomide and nanotechnology, applied in the field of amphiphilic block polymers, can solve the problems of inability to release drugs, increase the accumulation of drug carriers, reduce drug efficacy, etc., and achieve the effects of not easy dissociation, drug stability, and prolongation of half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
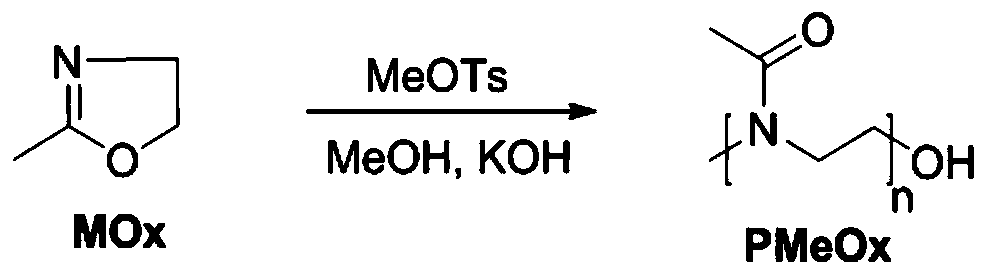
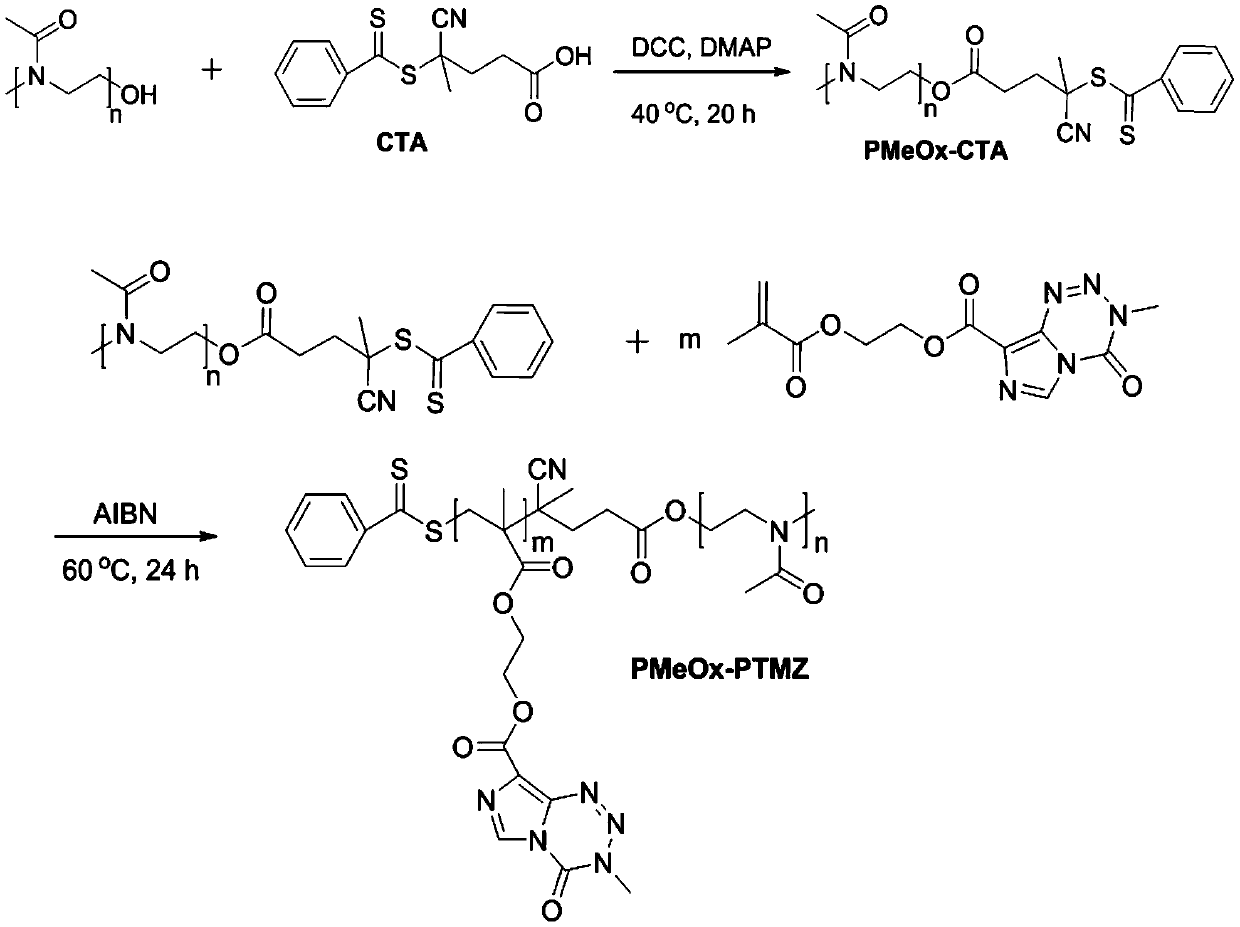
[0037] The synthesis method of polyoxazoline-polytemozolomide is similar except that the hydrophilic segment is different. Therefore, this embodiment only takes the synthesis of poly(2-methyl-2-oxazoline)-polytemozolomide as an example.
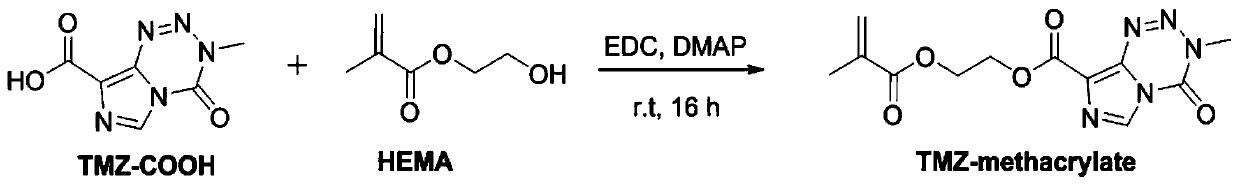
[0038] (1) Synthesis of temozolomide-methacrylate
[0039]
[0040]Dissolve 4.58g, 0.024mmol temozolomide (TMZ) in concentrated sulfuric acid, add 47.2mL of sodium nitrite aqueous solution dropwise under ice bath, keep stirring at room temperature for 17h and then drop to 0°C, add 122.0g of ice water to the system , to quench the reaction, filter, wash with ice water and dry to give temozolomide-8-carboxylic acid (TMZ-COOH).
[0041] Add 1.185g, 6.10mmol TMZ-COOH to the dichloromethane solution, then add 0.706mL of hydroxyethyl methacrylate (5.82mmol, HEMA), 1.348g of 1-(3-dimethylaminopropyl)- 3-Ethylcarbodiimide hydrochloride (7.02mmol, EDC) and 0.072g of 4-dimethylaminopyridine (0.58mmol, DMAP), continued to stir at room temperature fo...
Embodiment 2
[0062]
[0063] This embodiment is another preparation method of temozolomide nano-prodrug micelles (amphiphilic polyethylene glycol-polytemozolomide block polymer), and its preparation method is basically similar to that of Example 1, mainly comprising: anhydrous and oxygen-free Under the condition of environment, react polyethylene glycol (PEG) with 4-cyano-4-(thiobenzoyl)valeric acid (CTA) through esterification reaction, and then use PEG-CTA as macromolecular RAFT reagent, in Under the catalysis of azobisisobutyronitrile (AIBN), react with temozolomide-methyl methacrylate to obtain polyethylene glycol-polytemozolomide block polymer. The molecular weight of polyethylene glycol is 3000-10000Da, the molecular weight of polytemozolomide is 3000-10000Da, the degree of polymerization of polyethylene glycol is 39-131, and the degree of polymerization of polytemozolomide is 10-32.
Embodiment 3
[0065] Dissolve the hydrophobic drug in the organic solution first, then stir together with the organic solution of the amphiphilic block polymer, then add dropwise secondary water twice the volume of the organic solution, and stir the obtained solution for 1 hour Dialysis to obtain nanomicelles encapsulating the drug; the hydrophobic drug is selected from but not limited to: one of adriamycin, paclitaxel, methotrexate, curcumin or camptothecin.
[0066] The encapsulation of anticancer drugs in polyoxazolin-polytemozolomide micelles and polyethylene glycol-polytemozolomide micelles is all realized by dialysis. Taking PMeOx-PTMZ as an example, 2.4 mg of the polymer was dissolved in 1.0 mL of dimethyl sulfoxide, and the designed doxorubicin (DOX) required for a drug loading of 15% was added therein, and after ultrasonication for 0.5 h, Under the condition of stirring at room temperature, 1.8 mL of secondary water was slowly added dropwise to the dimethyl sulfoxide solution, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com