Preparation method of 7,8-dihydroxy flavone
A dihydroxyflavone and compound technology, applied in the field of preparation of 7,8-dihydroxyflavone, can solve the problems of 7,8-DHF industrial production limitation, high price, etc., and achieve easy industrial implementation, simple operation and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
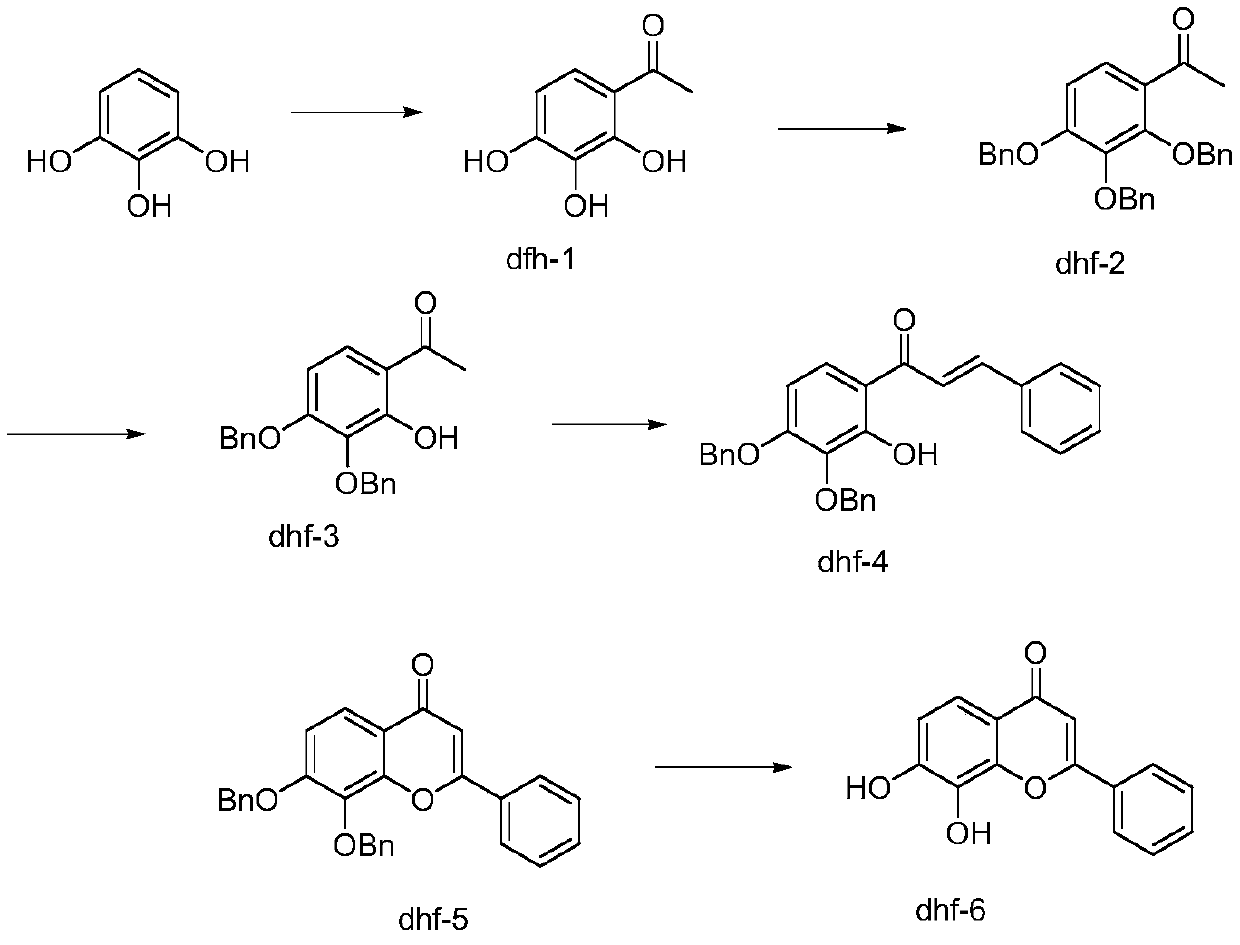
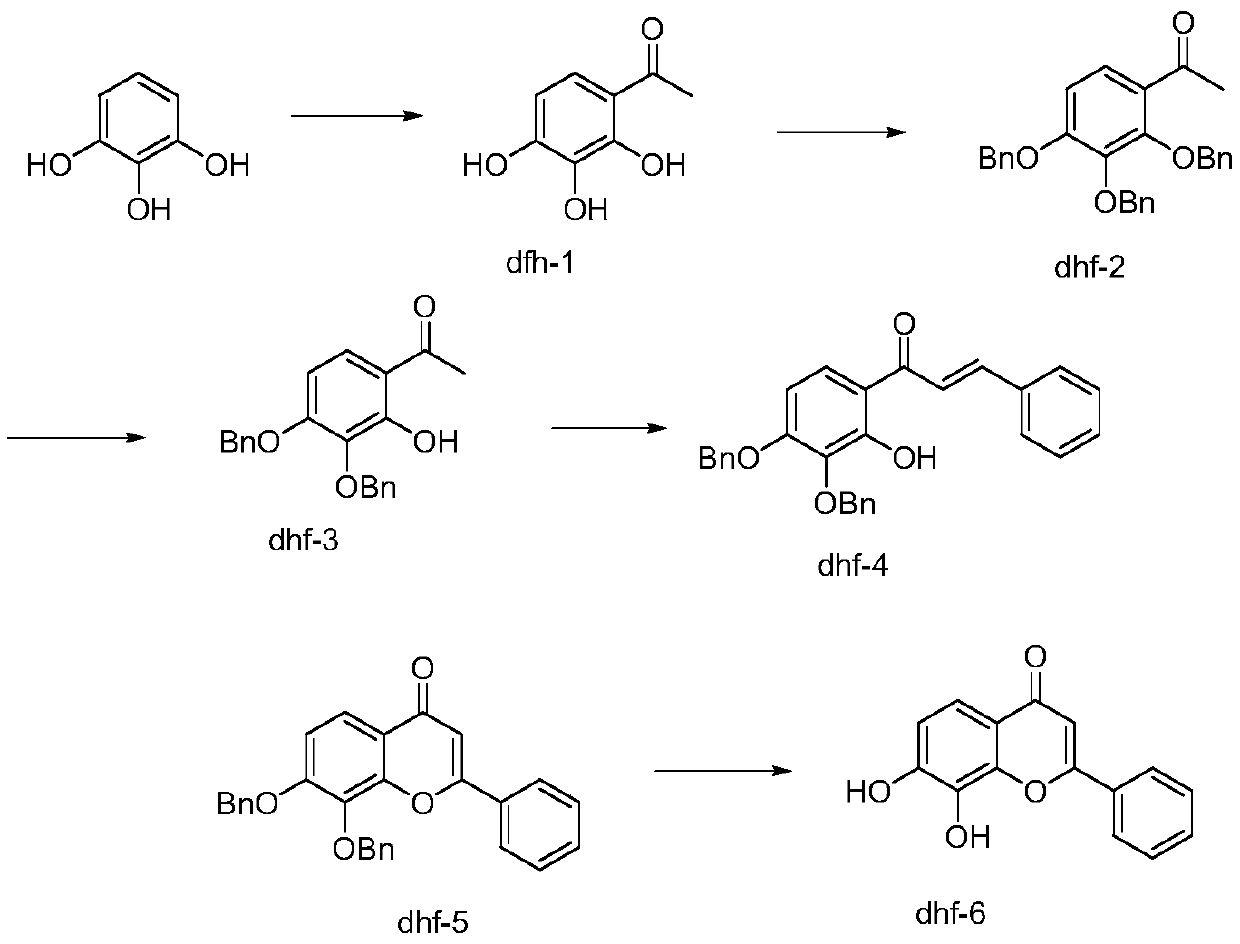
[0028] A kind of preparation method of 7,8-dihydroxyflavone described in this specific embodiment, its steps are as follows:
[0029] 1. Pyrogallol uses boron trifluoride ether as a solvent, acetic acid as an acylating reagent, undergoes Friedel-Crafts acylation reaction under the protection of an inert gas at a relatively low temperature, and the reactant is filtered, washed with alkali, and dried by suction to obtain Compound dhf1. Among them, the molar ratio of pyrogallol, acetic acid and boron trifluoride ether is 1:6-9:2-4.
[0030] 2. The compound dhf1 was mixed with benzyl chloride under the protection of an inert gas, potassium iodide was added, dbu was added dropwise to react at 110°C, the reaction solution was poured into ice water and stirred, and solids were precipitated, filtered by suction, washed with water and dried to obtain the compound dhf2 , The molar ratio of dhf1 to DBU and benzyl chloride is 1:5-7:5-7.
[0031] 3. The compound dhf2 is mixed with acetic...
Embodiment 1
[0036] A preparation method of 7,8-dihydroxyflavone, the steps are as follows:
[0037] (1) Synthesis of compound dhf1
[0038] Put 600g of glucinol and 2L of acetic acid into a 5L three-necked bottle, under the protection of Ar, control the temperature below 50°C, add 1.8L of boron trifluoride diethyl ether dropwise, and control the temperature at 90-100°C for 6-8h. After the reaction was completed, the crude product was directly obtained by suction filtration, and the crude product was washed with saturated sodium bicarbonate. Suction filtration and drying yielded 8 kg of a brownish-yellow solid product with a melting point of 63-64°C. The product was confirmed by NMR with a yield of 90%.
[0039] (2) Synthesis of compound dhf2
[0040] Under the protection of argon, put 1kg dhf-1 and 3kg benzyl chloride into a 5L three-necked bottle, add 50g of catalytic amount of potassium iodide dropwise to the mixed solution, and then add 3.6kg dbu dropwise to it under the control temp...
Embodiment 2
[0050] A preparation method of 7,8-dihydroxyflavone, the steps are as follows:
[0051] (1) Synthesis of compound dhf1
[0052] Put 600g of glucinol and 2L of acetic acid into a 5L three-neck bottle, under the protection of Ar, control the temperature below 60°C, add 1.9L of boron trifluoride diethyl ether dropwise, and control the temperature at 90°C for 6h. After the reaction was completed, the crude product was directly obtained by suction filtration, and the crude product was washed with saturated sodium bicarbonate. After suction filtration and drying, 8.5 kg of a brownish-yellow solid product with a melting point of 63° C. was obtained, and the product was confirmed by NMR with a yield of 89.50%.
[0053] (2) Synthesis of compound dhf2
[0054] Under the protection of argon, put 1kg dhf-1 and 3kg benzyl chloride into a 5L three-necked flask, add 50g of catalytic amount of potassium iodide dropwise to the mixed solution, and then add 4.0kg dbu dropwise to the mixed solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com