Method for preparing branched polyhydroxyethyl methacrylate by inverse emulsion polymerization at room temperature
A technology of branched poly(hydroxyethyl methacrylate) and inverse emulsion polymerization, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc. Long time and other problems, to achieve the effect of simple and stable reaction system, high monomer conversion rate and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
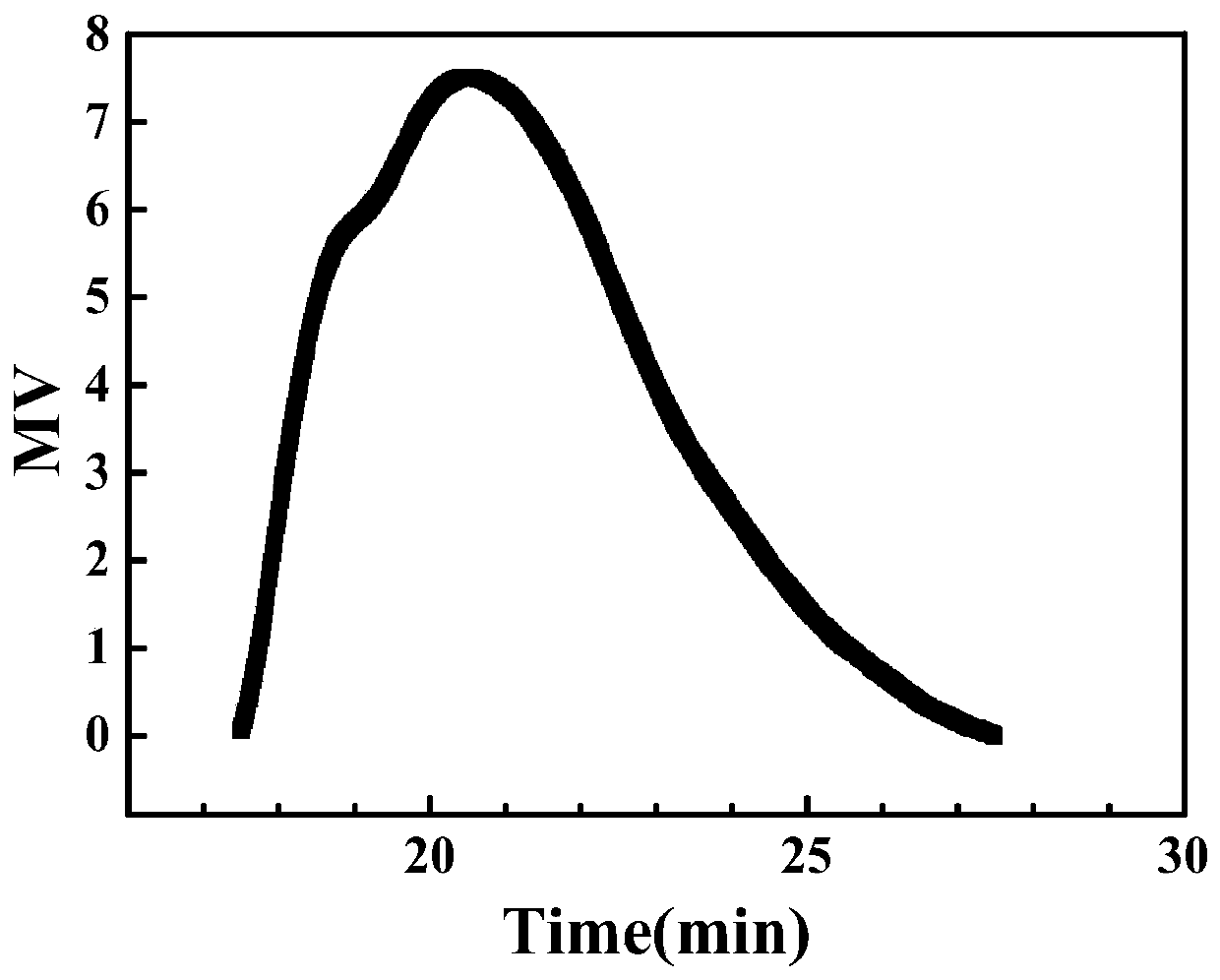
[0025] Hydroxyethyl methacrylate (10.4112g, 0.0800mol) was added into water (31.2336g, 300wt% hydroxyethyl methacrylate) to dissolve, and an aqueous solution of hydroxyethyl methacrylate (25wt%) was configured. The aqueous solution of hydroxyethyl methacrylate was added to a mixture of toluene (41.6448g, 400wt% hydroxyethyl methacrylate), Span85 (2.0822g, 5wt% toluene), Tween80 (2.0822g, 5wt% toluene), reducing agent single MPAEMA (0.2320g, 0.0010mol) in a reaction flask, stirred evenly and evacuated oxygen, then added the oxidant BPO (0.2422g, 0.0010mol), placed in a water bath at 25°C for 8 hours, measured methacrylic acid The conversion rate of hydroxyethyl ester was 94.3%. Demulsify with tetrahydrofuran, wash with water three times and then dry, then purify three times and then dry to obtain polymer. The polymer is analyzed by dynamic and static light scattering and Ubbelohde viscometer, the results are as follows: absolute weight average molecular weight M w.MALLS =2442...
Embodiment 2
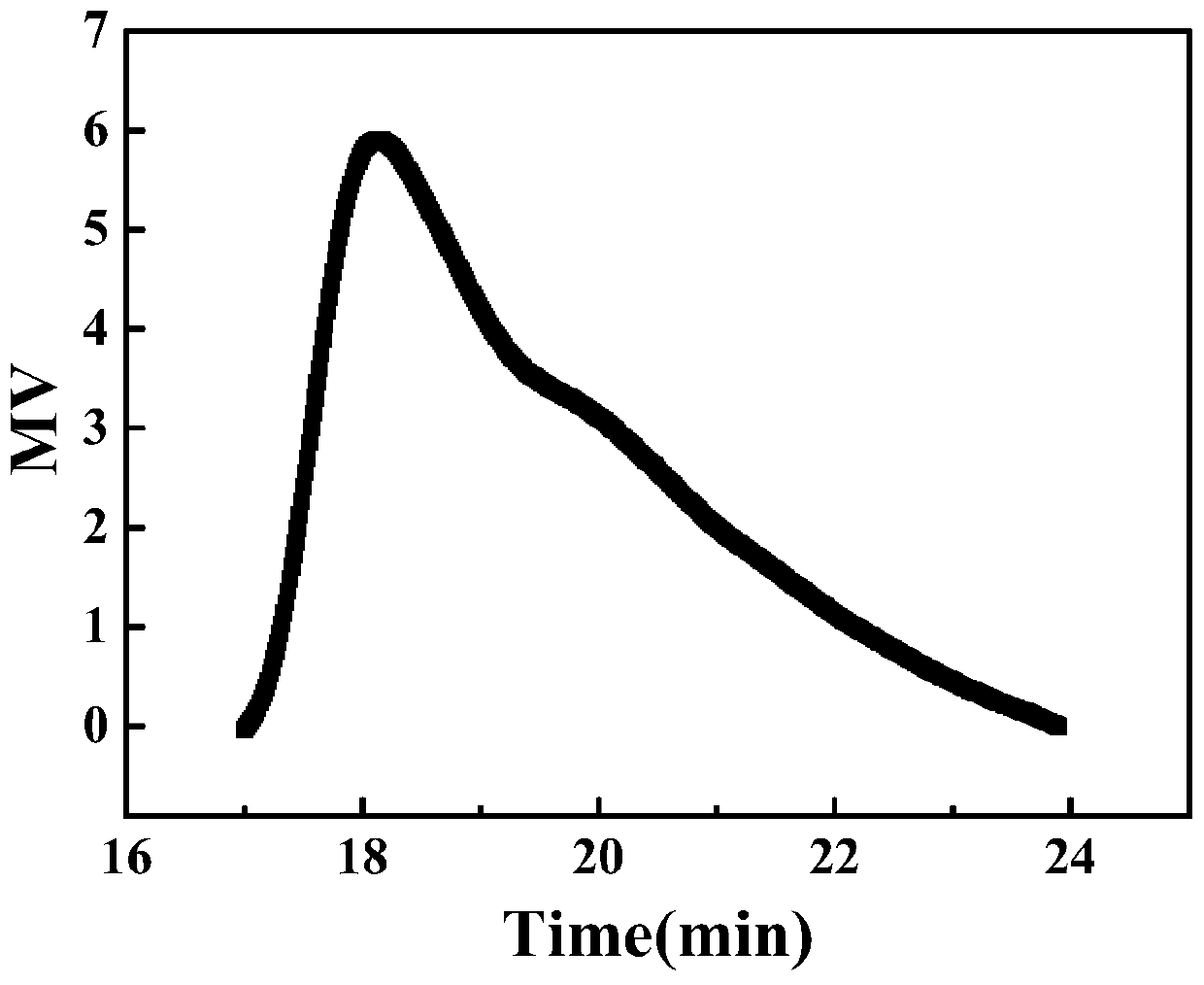
[0030] Hydroxyethyl methacrylate (13.0140g, 0.1000mol) was added into water (39.0420g, 300wt% hydroxyethyl methacrylate) to dissolve, and an aqueous solution of hydroxyethyl methacrylate (25wt%) was configured. The aqueous solution of hydroxyethyl methacrylate was added to a mixture of toluene (52.0560g, 400wt% hydroxyethyl methacrylate), Span85 (2.6028g, 5wt% toluene), Tween80 (2.6028g, 5wt% toluene), reducing agent single MPAEMA (0.2320g, 0.0010mol) in a reaction flask, stirred evenly and evacuated oxygen, then added the oxidant BPO (0.2422g, 0.0010mol), placed in a water bath at 25°C for 8 hours, measured methacrylic acid The conversion rate of hydroxyethyl ester was 93.7%. Demulsify with tetrahydrofuran, wash with water three times and then dry, then purify three times and then dry to obtain polymer. The polymer is analyzed by dynamic and static light scattering and Ubbelohde viscometer, the results are as follows: absolute weight average molecular weight M w.MALLS =2083...
Embodiment 3
[0033]Hydroxyethyl methacrylate (15.6168g, 0.1200mol) was added into water (46.8504g, 300wt% hydroxyethyl methacrylate) to dissolve, and an aqueous solution of hydroxyethyl methacrylate (25wt%) was configured. The aqueous solution of hydroxyethyl methacrylate was added to a mixture of toluene (62.4672g, 400wt% hydroxyethyl methacrylate), Span85 (3.1234g, 5wt% toluene), Tween80 (3.1234g, 5wt% toluene), reducing agent single MPAEMA (0.2320g, 0.0010mol) in a reaction flask, stirred evenly and evacuated oxygen, then added the oxidant BPO (0.2422g, 0.0010mol), placed in a water bath at 25°C for 8 hours, measured methacrylic acid The conversion rate of hydroxyethyl ester was 94.5%. Demulsify with tetrahydrofuran, wash with water three times and then dry, then purify three times and then dry to obtain polymer. The polymer is analyzed by dynamic and static light scattering and Ubbelohde viscometer, the results are as follows: absolute weight average molecular weight M w.MALLS =16710...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com