Ultrasonic activateable medicine-release albumin nanoparticles as well as preparation method and application thereof
A nanoparticle and albumin technology, applied in the field of biomedical new materials, can solve the problems of drug release rate of less than 20%, uncontrollable rapid drug release, and low drug release, so as to avoid toxicity and facilitate large-scale preparation , good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of Molecular Active Ester of Chemical Crosslinker
[0051] Weigh 50 mg of 4,4'-azobis(4-cyanovaleric acid) into a 25 mL flask, add 2 mL of anhydrous dimethyl sulfoxide to it, and magnetically stir until completely dissolved. Under stirring conditions, 50 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 80 mg of N-hydroxysuccinimide were successively added until completely dissolved. After reacting for two hours at 37°C, a colorless and clear 4,4'-azobis(4-cyanovaleric acid) active ester solution was obtained.
Embodiment 2
[0053] Preparation of albumin nanoparticles activated by ultrasound
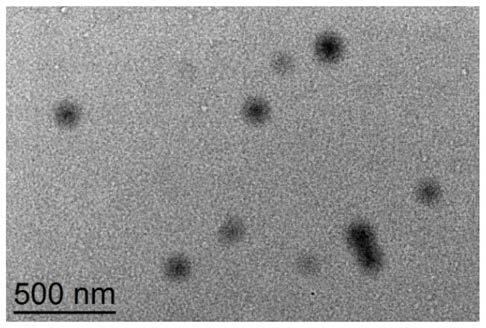
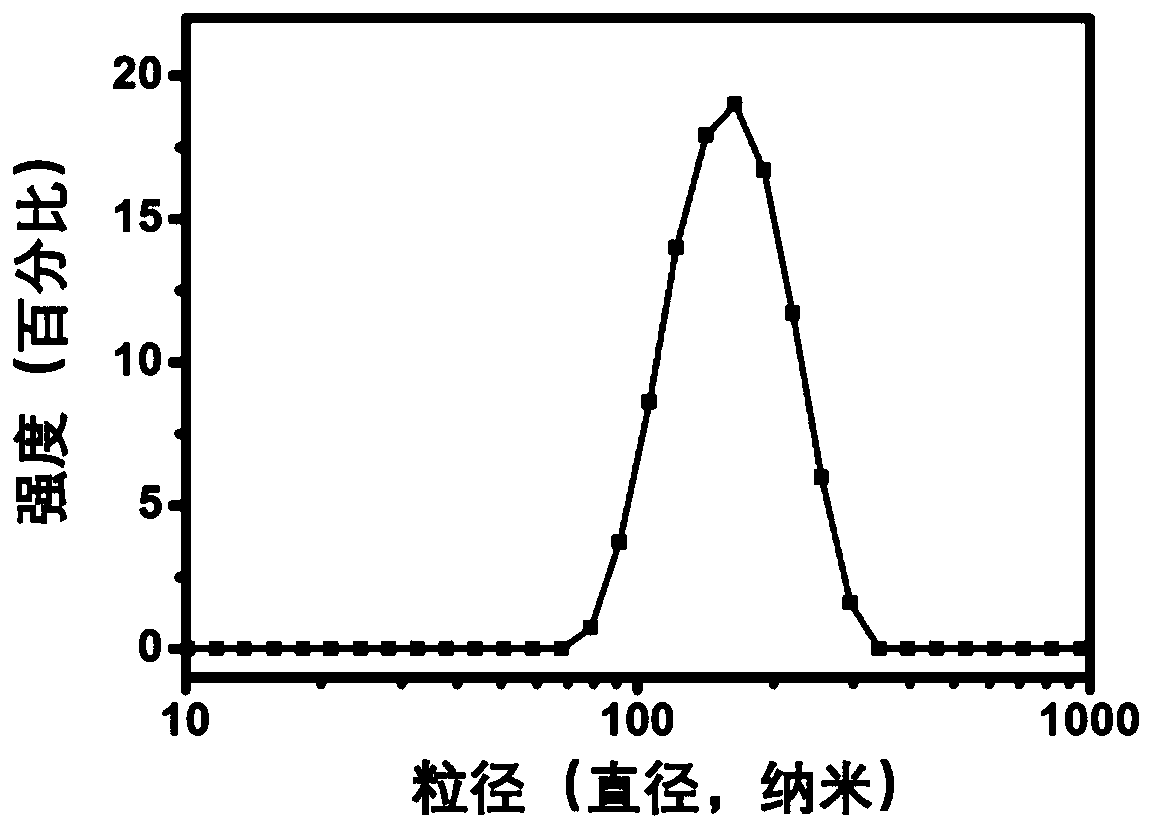
[0054] Bovine serum albumin-based nanoparticles (BSA-ACVA) were prepared by a desolvation-chemical cross-linking method. In principle, weigh 90 mg of bovine serum albumin powder and dissolve in 3 mL of ultrapure water. Add 0.1mol / L sodium hydroxide aqueous solution dropwise, adjust the pH value of the solution to 9.0, continue stirring for one hour, add 6mL absolute ethanol dropwise at a constant speed of 4mL / min, after the desolvation process is completed, then slowly add 80 μL of the linker solution was cross-linked and solidified, and the reaction was continued overnight at 37°C in the dark to obtain a stable albumin nanoparticle solution. Rotate the obtained albumin nanosuspension at 37°C to remove ethanol, centrifuge at 12000rpm, wash the precipitate with deionized water, remove uncrosslinked albumin and excess crosslinking agent with deionized water, repeat three times, Finally, disperse with a certa...
Embodiment 3
[0057] Preparation of Ultrasound-Activatable Drug Albumin Nanoparticles
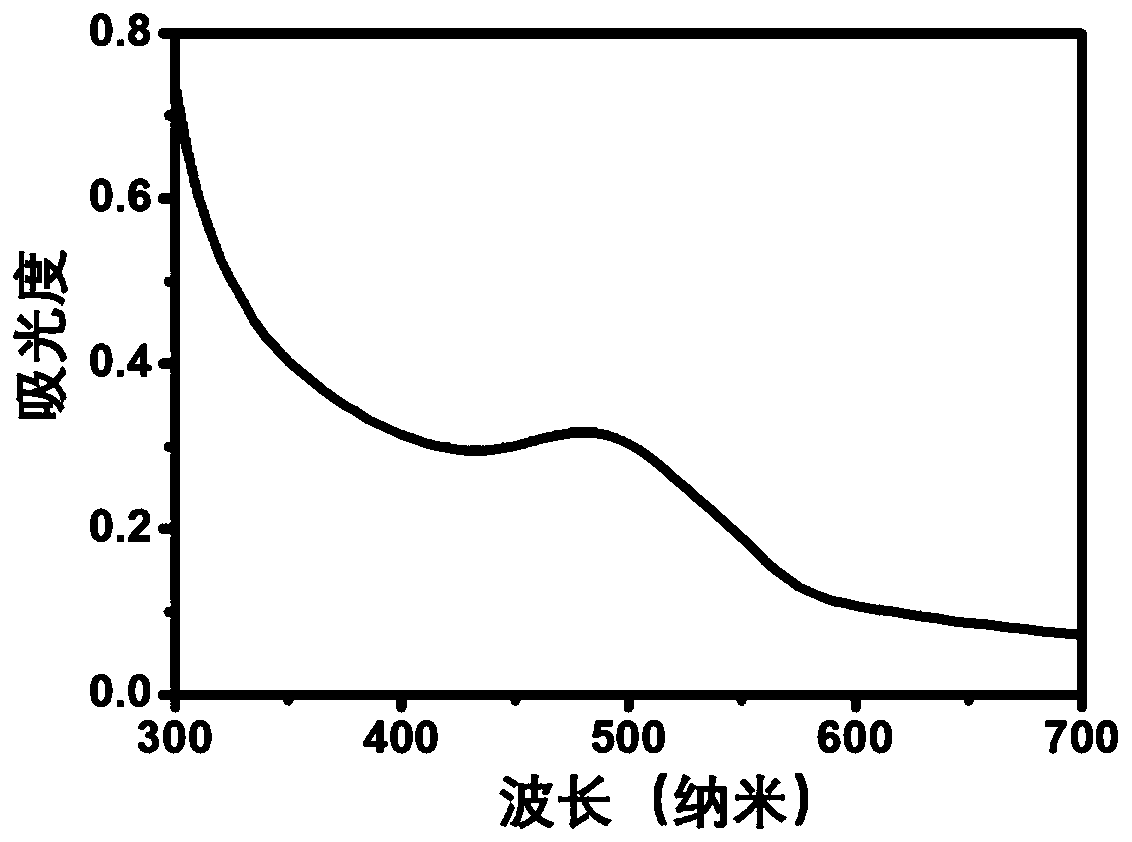
[0058] Drug-loaded bovine serum albumin nanoparticles (BSA-ACVA-DOX) were prepared by desolvation-chemical cross-linking method. Weigh 90 mg of bovine serum albumin powder and dissolve it in 3 mL of ultrapure water, add 6.0 mg of doxorubicin hydrochloride powder, and stir until completely dissolved. Add 0.1mol / L sodium hydroxide aqueous solution dropwise, adjust the pH value of the solution to 9.0, continue stirring for one hour, add 6mL absolute ethanol dropwise at a constant speed of 4mL / min, after the desolvation process is completed, then slowly add 80 μL of the linker solution was cross-linked and solidified, and the reaction was continued overnight at 37°C in the dark to obtain a stable albumin nanoparticle solution. Rotate the obtained albumin nanosuspension at 37°C to remove ethanol, centrifuge at 12000rpm, wash the precipitate with deionized water, remove uncrosslinked albumin and excess crossl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com