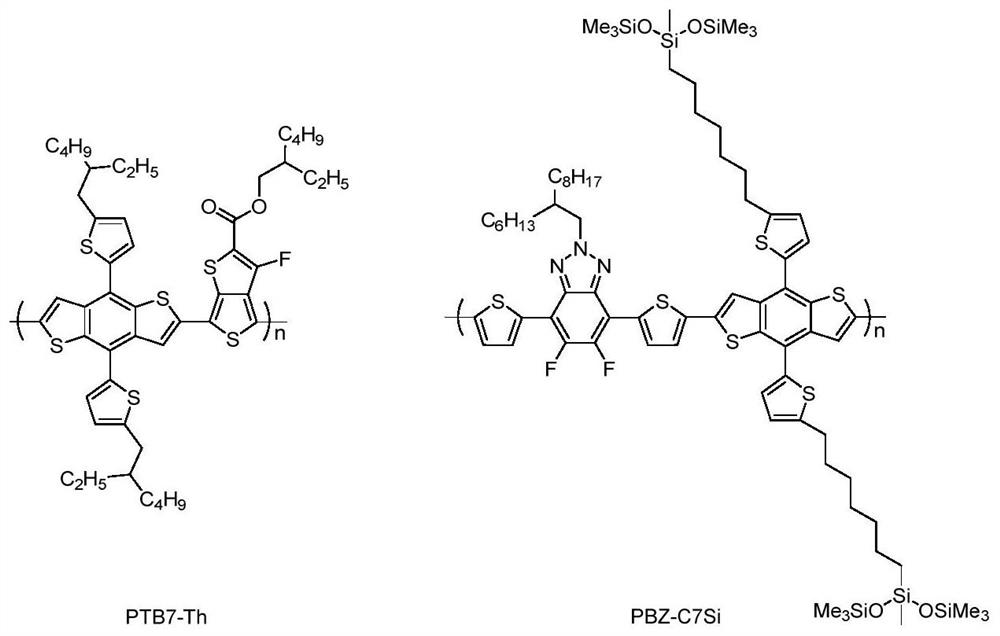
A class of aromatic condensed rings substituted by siloxane group and its preparation method and application
A siloxane-based, aromatic technology with applications in chemical instruments and methods, silicon organic compounds, semiconductor/solid-state device manufacturing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
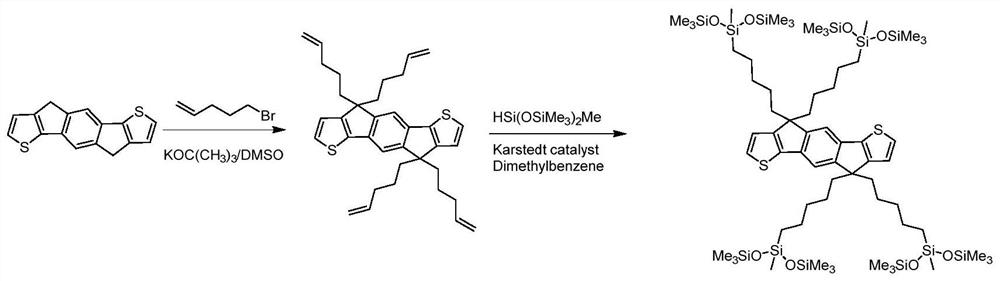
[0067] Example 1: Aromatic fused ring compound M substituted by siloxane group 1 Synthesis
[0068] m 1 The synthetic route of figure 2 shown. Concrete reaction steps and reaction conditions are as follows:
[0069]
[0070] Under nitrogen protection, A (2.66g, 10mmol) was dissolved in anhydrous dimethyl sulfoxide (50ml), potassium tert-butoxide (8.98g, 80mmol) was added three times, and reacted at 80°C for 1 hour. Then 5-bromopent-1-ene (11.92, 80 mmol) was added dropwise, and reacted at 85-90° C. for 12 hours. The reaction was quenched by adding water, extracted three times with ethyl acetate, and washed several times with distilled water. Using petroleum ether as the eluent, the product was further purified by silica gel chromatography to obtain a light yellow solid (3.23 g, yield 60%), namely compound C.
[0071] Under nitrogen protection, C (3.23g, 6mmol) and 1,1,1,3,5,5,5-heptamethyltrisiloxane (10.68g, 48mmol) were dissolved in anhydrous THF (50ml) , add 5 dr...
Embodiment 3
[0078] Embodiment 3: Formula M 3 The synthetic M of shown small organic molecule (i.e. specific compound shown in formula I ') 4 The synthetic route of Figure 4 shown. Concrete reaction steps and reaction conditions are as follows:
[0079]
[0080] Under nitrogen protection, compound D (2.32g, 1.32mmol) and compound N (1.69g, 5.30mmol) were placed in a two-necked flask, and dissolved in anhydrous o-xylene (30ml), and then catalyst four (three Phenylphosphine) palladium (0) (76.5mg, 0.067mmol), react at 120°C overnight. The reactant was cooled to room temperature, and the solvent was dried with a rotary evaporator. Using petroleum ether / dichloromethane mixture as eluent, the product was purified by silica gel chromatography to obtain dark red solid N (1.11 g, 44%).
[0081] Under nitrogen protection, compound K (500 mg, 0.26 mmol) and compound H (483 mg, 2.10 mmol) were dissolved in chloroform (30 ml), added pyridine (1 ml), heated to reflux, and reacted overnight. Sub...
Embodiment 4
[0082] Example 4: Determination of UV-Vis Absorption of Molecules Using UV-Vis Spectrometer
[0083] For the small molecule M prepared in embodiment 2,3 2 , M 3 Chloroform solution and film absorption tests were performed. UV-Vis absorption spectrum such as Figure 5 shown.
[0084] m 2 , M 3 The optical absorption data are shown in Table 1. Wherein, a) is measured in the chloroform solution; b) is measured under the film state,
[0085]
[0086] As can be seen from the table, the small molecule M 2 The solution absorbs at 600-900nm, and the maximum molar extinction coefficient is 1.86×10 5 m -1 cm -1 ; The absorption red shift in the thin film state ranges from 600 to 1000nm, the absorption edge of the film reaches 1022nm, and the corresponding optical band gap is 1.21eV. It is a typical near-infrared small molecule and can be used as a narrow band gap acceptor in photovoltaic devices. small molecule M 3 The absorption range of the solution is in the range of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com