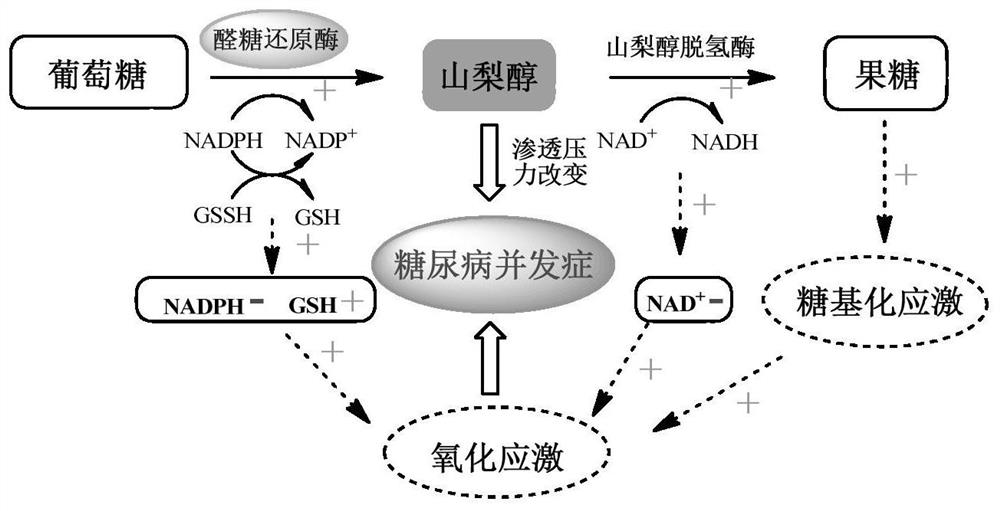
A kind of tetrazolium substituted quinolinone derivative and its preparation method and application
A technology of tetrazolium and quinolinone, applied in the field of organic compound preparation, can solve problems such as low activity in vivo, toxic and side effects, failure to pass FDA certification, etc., so as to prevent or prevent diabetic complications, treat diabetic complications, improve The effect of in vivo availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In this example, R 1 is tetrazolium; R 2 and R 3 for the hydrogen atom. i.e. compound 1 1-Benzyl-3-(1H-tetrazol-5-yl)quinolin-4(1H)-one.
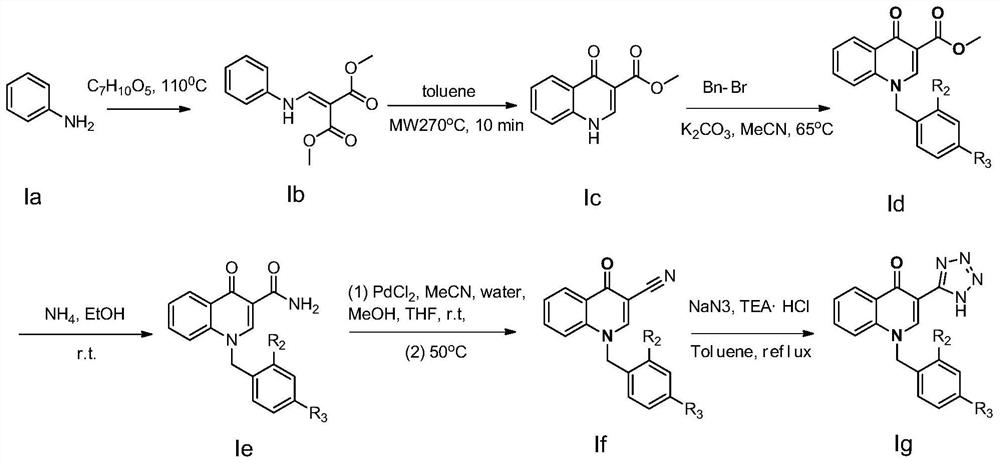
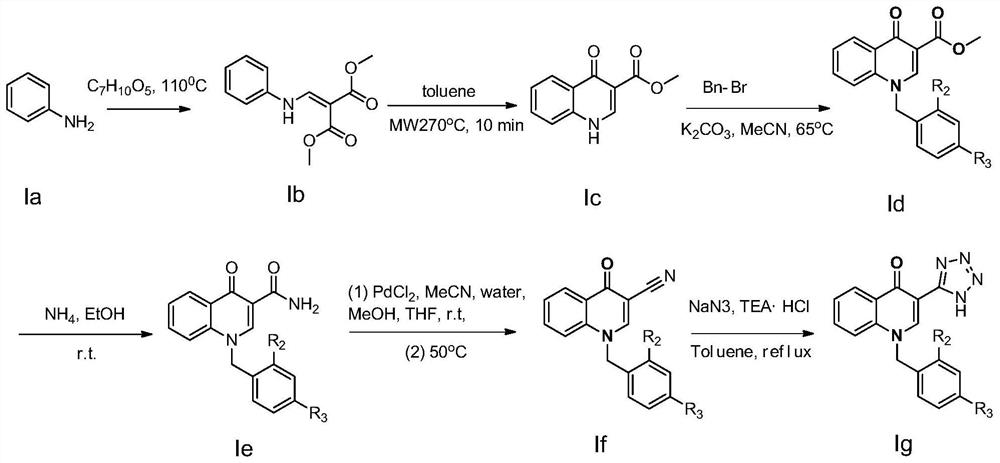
[0033] see figure 2 , the preparation method of above-mentioned 1-benzyl-3-(1H-tetrazol-5-yl) quinoline-4(1H)-one is as follows:
[0034] Step 1. add 20mmol aniline (compound Ia in the four-necked flask) ) and 30mmol of dimethyl methoxymethylene maleate, the reaction mixture was carried out Neat reaction at 110 ° C, TLC (thin layer chromatography) was monitored until aniline disappeared, after the reaction system was cooled, ether was added dropwise to the system to precipitate Generated, after stirring for 20min, filtered to obtain a solid crude product, the crude product was purified by beating with n-heptane (5vol. (solvent volume, 5 times the volume of the crude product weight (5mL / g crude product weight))) to obtain a white solid compound Ib (ie 2-[(phenylamino)methylene]-1,3-dimethyl ester (4.02 g, yield 85.5%): melt...
Embodiment 2
[0041] In this example, R 1 For tetrazolium, R 2 is a hydrogen atom, R 3 is a fluorine atom. i.e. compound 2 1-(4-Fluorobenzyl)-3-(1H-tetrazol-5-yl)quinolin-4(1H)-one.
[0042] The preparation method of the above-mentioned 1-(4-fluorobenzyl)-3-(1H-tetrazol-5-yl)quinoline-4(1H)-one is the same as that in Example 1, except that 4-fluorobenzyl bromide is used instead of Benzyl bromide in Example 1. The compound 2,1-(4-fluorobenzyl)-3-(1H-tetrazol-5-yl)quinolin-4(1H)-one was finally obtained (melting point: 217-219°C; 1 H NMR (400MHz, [D 6 ]DMSO): δ / ppm=14.19(s, 1H), 8.50(s, 1H), 8.39(dd, J=8.0, 1.6Hz, 1H), 7.46(dd, J=8.2, 1.6Hz, 1H), 7.45-7.52(m, 4H), 7.26-7.18(m, 2H), 5.47(s, 2H); 13 C NMR (100MHz, [D 6 [M+Na] + ).
Embodiment 3
[0044] In this example, R 1 For tetrazolium, R 2 is a hydrogen atom, R 3 for the chlorine atom. i.e. compound 3 1-(4-Chlorobenzyl)-3-(1H-tetrazol-5-yl)quinolin-4(1H)-one.
[0045] The preparation method of above-mentioned 1-(4-chlorobenzyl)-3-(1H-tetrazol-5-yl)quinoline-4(1H)-one is the same as that in Example 1, except that 4-chlorobenzyl bromide is used instead of Benzyl bromide in Example 1. The compound 3,1-(4-chlorobenzyl)-3-(1H-tetrazol-5-yl)quinolin-4(1H)-one was finally obtained (melting point: 215-217°C; 1 H NMR (400MHz, [D 6 ]DMSO): δ / ppm=14.17(s, 1H), 8.47(s, 1H), 8.41(dd, J=7.8, 1.6Hz, 1H), 7.44(dd, J=8.0, 1.6Hz, 1H), 7.51-7.54(m, 4H), 7.31-7.25(m, 2H), 5.44(s, 2H); 13C NMR (100MHz, [D 6 ]DMSO)δ / ppm=177.1,168.4,151.4,141.7,139.2,137.9,131.7,130.8,129.5,127.4,125.2,123.4,115.6,109.4,58.7.MS m / z:360.1[M+Na] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com