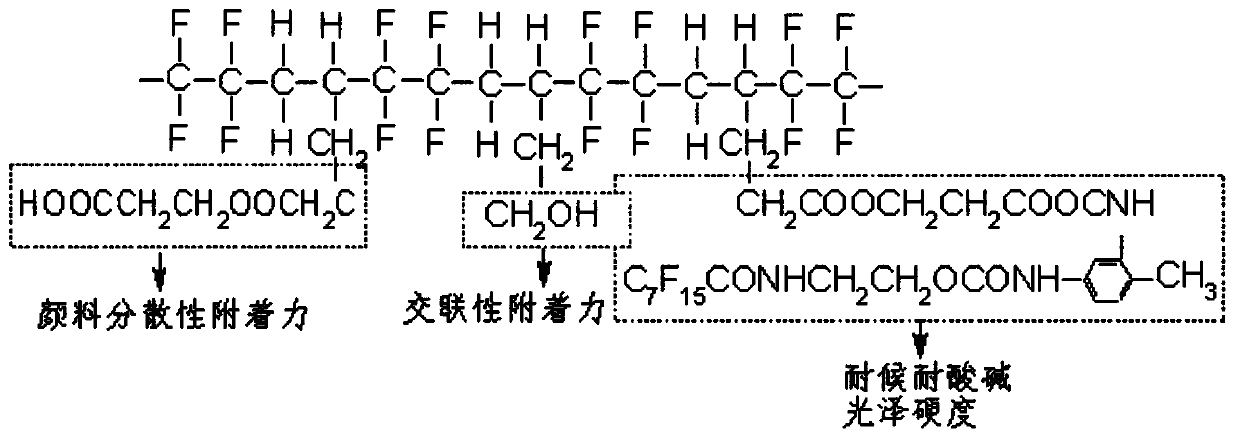
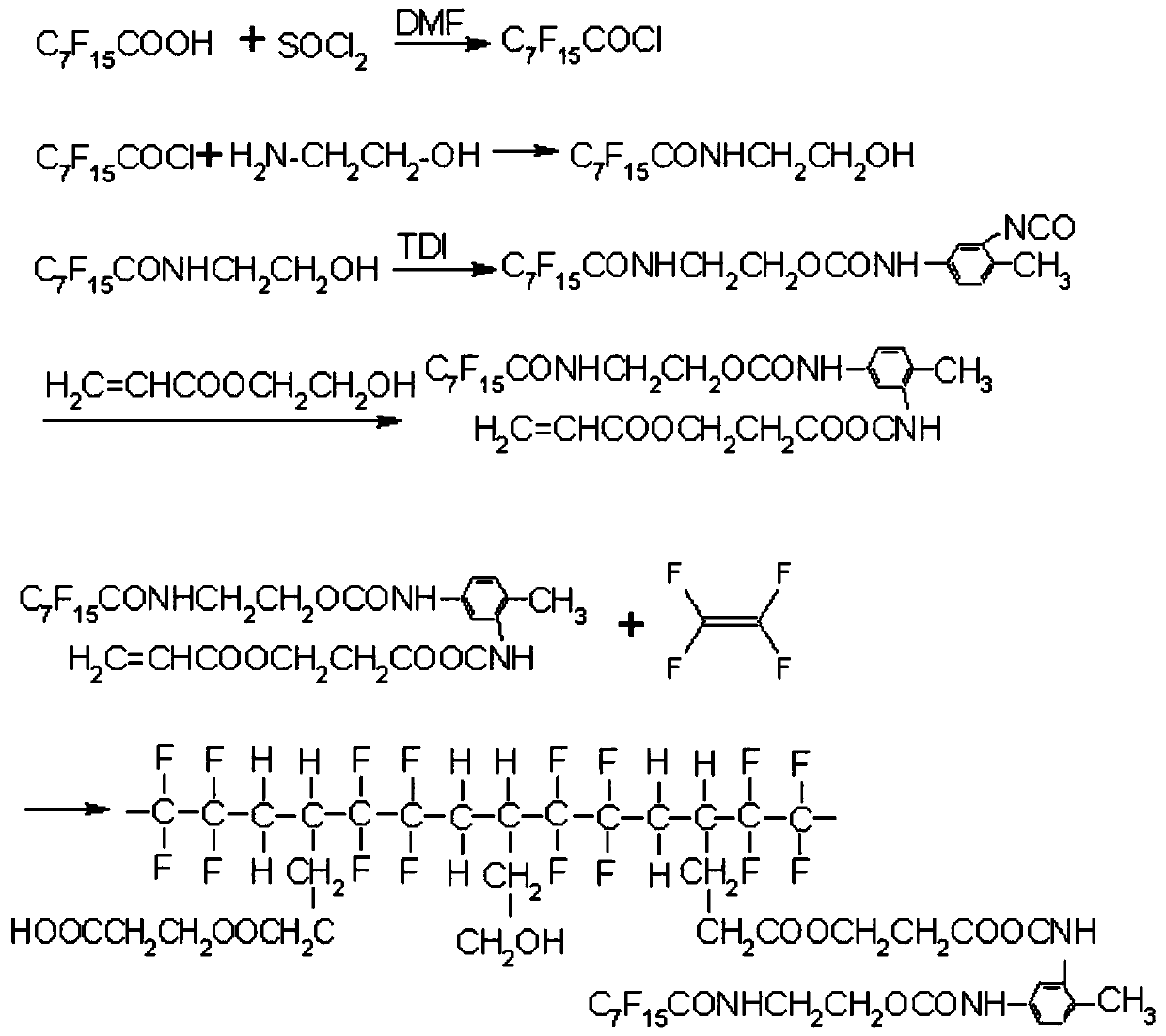
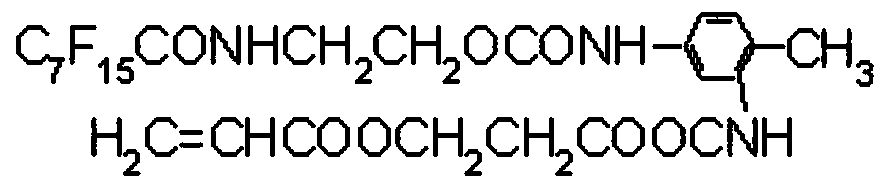Vinyl modified fluorocarbon resin, preparation method thereof, and corrosion-resistant coating and preparation method and application thereof
A kind of fluorocarbon resin and modified fluorocarbon technology, applied in the field of corrosion-resistant coatings and their preparation, vinyl-modified fluorocarbon resin and its preparation, which can solve the problems of poor weather resistance and poor corrosion resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The invention provides a preparation method of vinyl-modified fluorocarbon resin, which comprises the following steps: mixing vinyl monomer containing fluorine group, initiator and tetrafluoroethylene gas-phase monomer, and carrying out addition reaction to obtain ethylene Modified fluorocarbon resin.
[0035] The present invention has no special limitation on the preparation method of the fluorocarbon monomer containing perfluorinated groups, and the preparation method well known to those skilled in the art can be used. In the present invention, the vinyl group of the fluorine-containing group The preparation method of monomer preferably comprises the following steps:
[0036] (1) Mix perfluorooctanoic acid, thionyl chloride and N,N-dimethylformamide, and perform the first esterification reaction to obtain perfluorooctanoyl chloride;
[0037] (2) Mixing the perfluorooctanoyl chloride, ethanolamine and tetrahydrofuran for a second esterification reaction to obtain N-hy...
Embodiment 1
[0081] Preparation raw materials and manufacturers are:
[0082] Perfluorooctanoic acid Shanghai Sanaifu New Material Co., Ltd.;
[0083] Thionyl Chloride Nanjing Rundi Industrial Co., Ltd.;
[0084] β-hydroxyethyl methacrylate, 2,4-toluene diisocyanate, methyl methacrylate, ethyl acrylate and butyl acrylate, industrial grade, all produced by Sanki Chemical Co., Ltd.;
[0085] Ethanolamine, dibutyltin dilaurate, N,N-dimethylformamide, methyl isobutyl ketone, chloroform, ethanol, acetone, NaOH, ethylene acid monomer and azobisisobutyronitrile, analytically pure, from Chengdu Branch Produced by Long Chemical Co., Ltd.
[0086] Add 15g of perfluorooctanoic acid and 9g of thionyl chloride into a four-necked flask equipped with a thermometer, a stirrer and a reflux condenser, add 0.1g of N,N-dimethylformamide as a catalyst, and carry out esterification reaction at 85°C 1h, gas chromatograph analysis, reach the end point when there is a stable value; reduce excess thionyl chlorid...
Embodiment 2
[0091] The preparation method of the vinyl monomer of fluorine-containing group is identical with embodiment 1;
[0092] Add 300g of fluorine-containing group vinyl monomer and 180g of initiator into the reaction kettle with stirring and electric heating, and evacuate until the oxygen content-5 g / L, add 60g of tetrafluoroethylene gas-phase monomer to the reactor at one time, raise the temperature to 76°C, and react for 20h under stirring conditions. Vacuumize, replace with nitrogen, and discharge to obtain vinyl-modified fluorocarbon resin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Fineness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com