Flavone derivative for treating tumor and application thereof
A technology of derivatives and flavonoids, applied in the field of novel flavonoid derivatives and their pharmacologically acceptable salts, hydrates or solvates, can solve the problems of low bioavailability and poor solubility in water, and achieve good anti-tumor effects. Activity, high solubility, broad anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
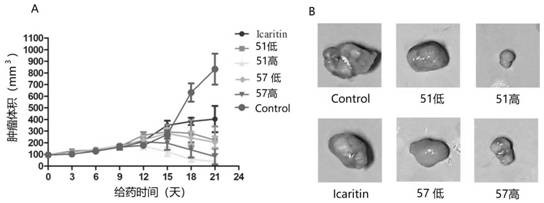
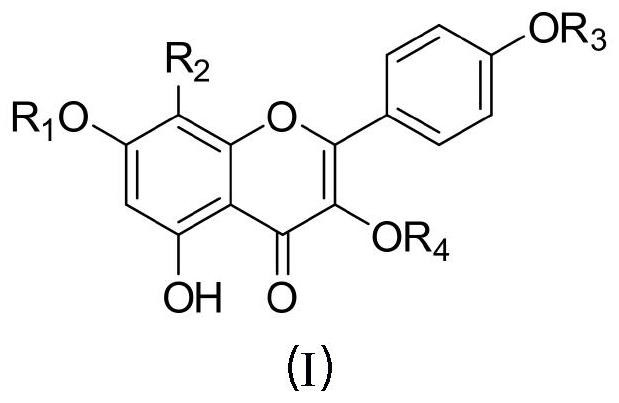
Image
Examples
Embodiment 1
[0060] The synthesis of embodiment 1 compound 35
[0061]
[0062]Weigh 9g of compound 25 and dissolve it in 60mL of dichloromethane, add 25mL of 33% hydrobromic acid in acetic acid solution, react at room temperature for 2 hours, TLC monitors that the reaction is complete, pour it into 50mL of water, extract with dichloromethane (50mL×3), and combine The organic layer was washed once with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, filtered, concentrated, and recrystallized with a mixed solvent of petroleum ether / ethyl acetate 10 / 1 to obtain 8.2 g of compound 26 with a yield of 86.7%.
[0063] Dissolve 8.2g of compound 26 in 40mL of dry DMSO, add 1.56g of sodium azide, stir at room temperature for 1 hour, TLC monitors that the reaction is complete, pour it into 100mL of water, extract with ethyl acetate (50mL×3), combine the organic layers and use After drying over anhydrous sodium sulfate, filtering, and concentrating, 5.8 g of compound 27 was obtai...
Embodiment 2
[0071] The synthesis of embodiment 2 compound 39
[0072]
[0073] Weigh 18g of compound 25 and dissolve it in 150mL of dichloromethane, add 6.3g of bromoethanol and 8.7mL of boron trifluoride diethyl ether, and react overnight at room temperature. Saturated sodium bicarbonate was added until no gas was generated, extracted three times with DCM, the organic layers were combined and dried, concentrated, and column chromatography gave 10 g of compound 36, with a yield of 47.7%.
[0074] 10 g of compound 36 was weighed and dissolved in 150 mL of DMF, and 2.86 g of sodium azide was added. React overnight at 60°C. TLC monitored the completion of the reaction. Cool to room temperature, add an appropriate amount of water, extract twice with ethyl acetate (100mL×3), concentrate to remove several layers and a small amount of residual DMF, add an appropriate amount of ethanol to precipitate a solid, and filter to obtain 6.9g of product 37, yield 75.2%.
[0075] Weigh 1g of compoun...
Embodiment 3
[0078] The synthesis of embodiment 3 compound 44
[0079]
[0080] Weigh 1.24g of compound 40 and add it to 15mL of water, add 1.95g of sodium azide and 150mg of sodium iodide in sequence, react at 60°C for 96 hours, cool to room temperature, add sodium chloride to saturation, extract with dichloromethane (20mL×3 ), the organic layers were combined and dried, and concentrated to obtain 900 mg of colorless liquid compound 41, with a yield of 68.7%.
[0081] Weigh 1.25g of compound 25 and 524mg of compound 41 and dissolve it in 20mL of dichloromethane, cool to 0°C, slowly add 0.6mL of boron trifluoride ether, after the addition is completed, slowly warm up to room temperature and react overnight at room temperature, slowly to 1 mL of triethylamine was added dropwise to the reaction system, concentrated in vacuo under reduced pressure, and passed through a column (PE:EA=20:1-2:1) to obtain 1.03 g of compound 42 with a yield of 70%.
[0082] Weigh 1g of compound 42 and dissolv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com