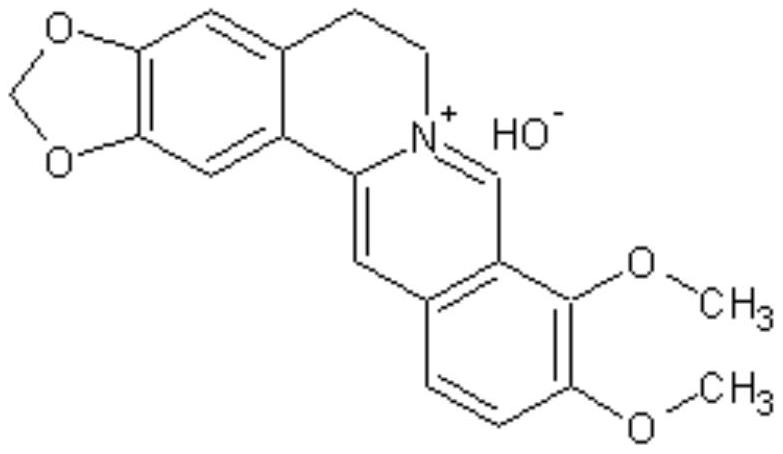
Application of berberine-oryzanol tablet in treatment of diabetes
A technology of berberine hydrochloride and oryzanol, which is applied in the field of medicine, can solve the problems of low bioavailability and drug dissolution, achieve the effects of simple prescription and preparation process, increase specific surface area, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
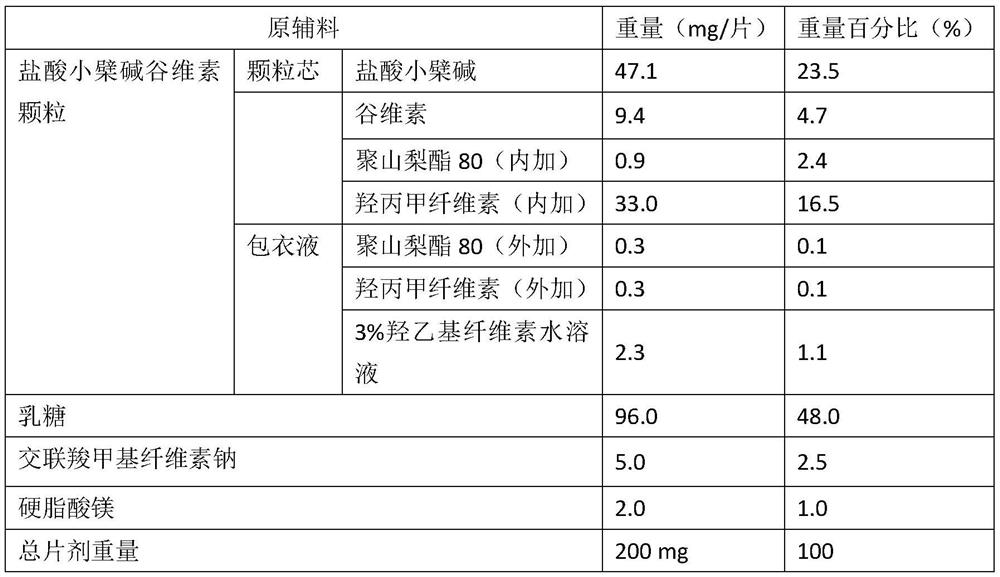
[0031] A kind of berberine hydrochloride oryzanol tablet, its composition is as follows:
[0032]
[0033] Preparation:
[0034] (1) Preparation of berberine hydrochloride oryzanol granules
[0035] Mix berberine hydrochloride, oryzanol, polysorbate 80, and hypromellose, jet mill, and dry granulate. The added polysorbate 80 and the added hypromellose were dissolved in 3% hydroxyethyl cellulose aqueous solution to obtain a coating solution. The above-mentioned coating liquid is sprayed onto the granules obtained by dry granulation by means of fluidized bed spraying, and the weight gain of the coating is 3%, to obtain the berberine hydrochloride oryzanol modified granules.
[0036] (2) Preparation of tablets
[0037] The above-mentioned berberine hydrochloride oryzanol modified granules are mixed with lactose, croscarmellose sodium and magnesium stearate and then compressed into tablets.
Embodiment 2
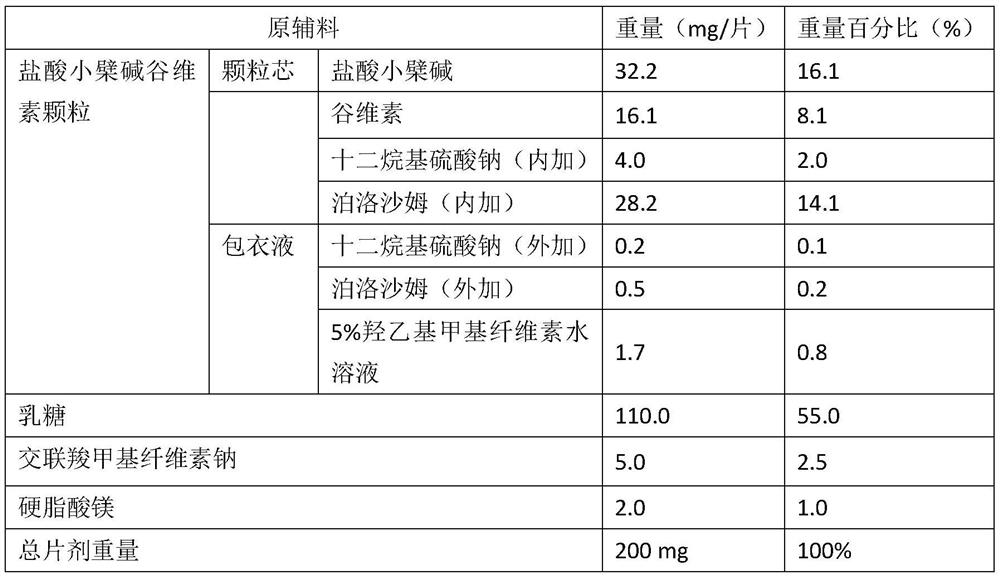
[0039] A kind of berberine hydrochloride oryzanol tablet, its composition is as follows:
[0040]
[0041] Preparation:
[0042] (1) Preparation of berberine hydrochloride oryzanol granules
[0043] Mix berberine hydrochloride, oryzanol, sodium lauryl sulfate, and poloxamer, jet mill, and dry granulate. Added sodium lauryl sulfate and added poloxamer were dissolved in 5% aqueous solution of hydroxyethyl methylcellulose to obtain a coating solution. The above-mentioned coating liquid is sprayed onto the granules obtained by dry granulation by means of fluidized bed spraying, and the weight gain of the coating is 3%, to obtain the berberine hydrochloride oryzanol modified granules.
[0044] (2) Preparation of tablets
[0045] The above-mentioned berberine hydrochloride oryzanol modified granules are mixed with lactose, croscarmellose sodium and magnesium stearate and then compressed into tablets.
Embodiment 3
[0047] A kind of berberine hydrochloride oryzanol tablet, its composition is as follows:
[0048]
[0049] Preparation:
[0050] (1) Preparation of berberine hydrochloride oryzanol granules
[0051] Mix berberine hydrochloride, oryzanol, sodium lauryl sulfate, and hypromellose, jet mill, and dry granulate. The added sodium lauryl sulfate and the added hypromellose were dissolved in a 3% aqueous solution of hydroxyethyl methylcellulose to obtain a coating solution. The above-mentioned coating liquid is sprayed onto the granules obtained by dry granulation by means of fluidized bed spraying, and the weight gain of the coating is 3%, to obtain the berberine hydrochloride oryzanol modified granules.
[0052] (2) Preparation of tablets
[0053] The above-mentioned berberine hydrochloride oryzanol modified granules are mixed with microcrystalline cellulose, lactose, crospovidone and magnesium stearate, and then compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com