Stent and application thereof
A matrix and drug technology, applied in the field of medical devices, can solve the problems of narrow urinary system pipeline, increased economic burden, damage repair or therapeutic effect, and achieve high treatment efficiency, less drug dosage and uniform release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] This embodiment provides a stent, including a stent matrix and a glucocorticoid-containing drug dispersed in the stent matrix, wherein the glucocorticoid-containing drug is mometasone furoate, the average particle size is 5000 mesh, and the material of the stent matrix is Silicone rubber, the crosslink density of silicone rubber is 5000g / mol.
[0097] This embodiment provides a preparation method for a stent, comprising: 10 parts by weight of a drug (mometasone furoate) and 30 parts by weight of silicone rubber (HTV medical silicone rubber, molecular weight 20-1 million), 1.2 parts by weight of a cross-linking agent ( Hydroxyl silicone oil), 1.2 parts by weight of catalyst (platinum), kneaded on a rubber mixer until uniform, vulcanized and crosslinked, and extruded into a support on an extruder.
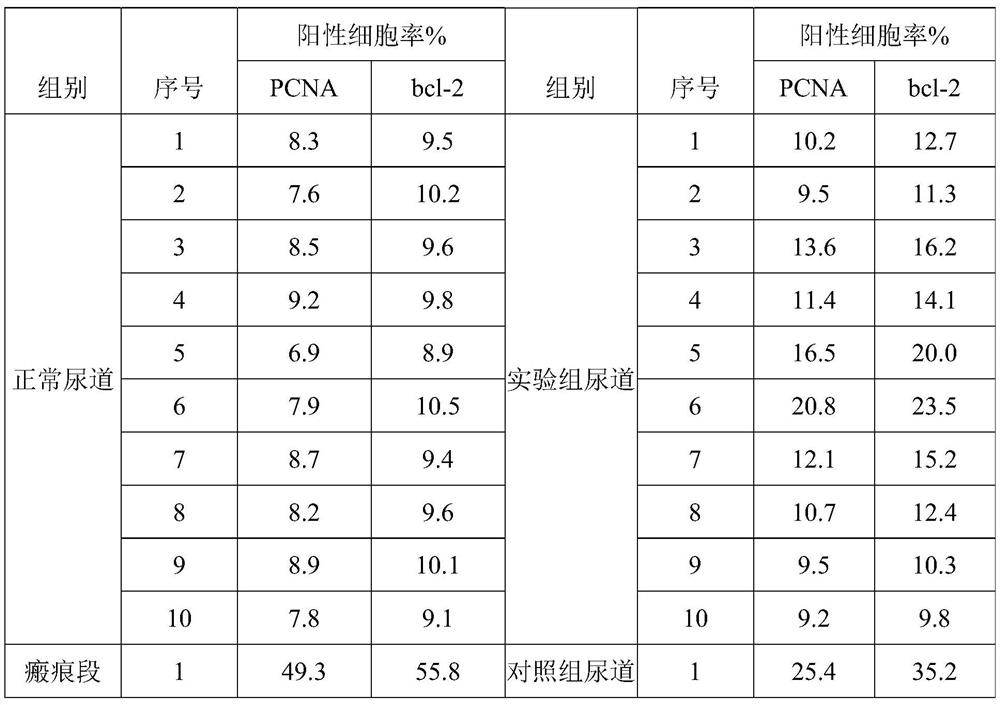
[0098] According to the test method of the standard GB / T531, the physical properties of the stent of this embodiment were tested, and it can be known that the hardness of the ...
Embodiment 2
[0147] This embodiment provides a stent, including a stent matrix and a drug containing a glucocorticoid dispersed in the stent matrix, wherein the material of the stent matrix is caprolactone-lactide copolymer, and the drug containing a glucocorticoid is budesel Germany.
[0148] This embodiment provides a method for preparing a stent, comprising: dissolving 20 parts by weight of a degradable material (caprolactone-lactide copolymer), 1.5 parts by weight of a drug (budesonide) containing a glucocorticoid in 50 ml of acetone , then add 2 parts by weight of water-soluble polymer (cellulose, cellulose is first dissolved in 5ml of water, and then added to the acetone solution) for mixing, the solvent is removed, heated and shaped to obtain a scaffold.
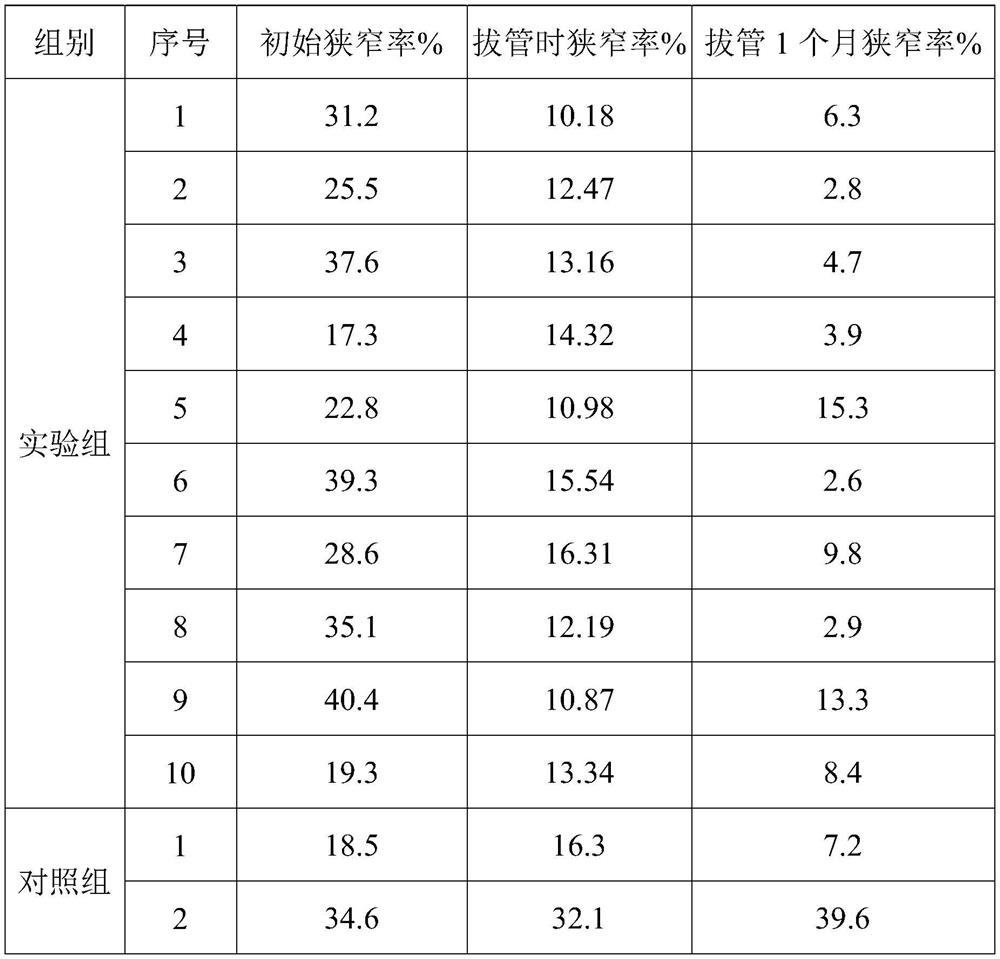
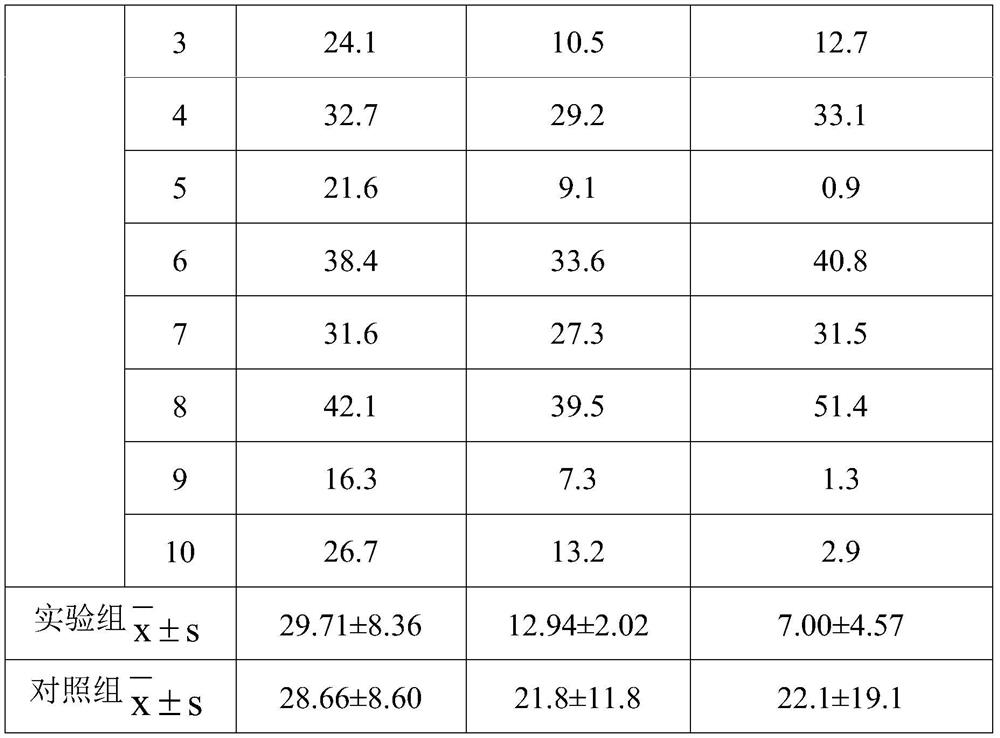
[0149] The support force of the stent was tested using a radial support dynamometer, and the force value of 10% compression was 92N.
[0150] Calculate the content of drug ingredients in the sample according to the weight, use ...
Embodiment 3
[0152] This embodiment provides a stent, including a stent matrix and a drug containing a glucocorticoid dispersed in the stent matrix, wherein the material of the stent matrix is polyglycolic acid, and the drug containing a glucocorticoid is mometasone furoate.
[0153] This embodiment provides a preparation method for a stent, comprising: 80 parts by weight of a degradable material (polyglycolic acid), 5 parts by weight of a drug containing a glucocorticoid (mometasone furoate) and 10 parts by weight of a water-soluble polymer ( Chitosan) was uniformly mixed under melting conditions (140° C.), extruded and shaped to obtain a scaffold.
[0154] The support force of the stent was tested using a radial support dynamometer, and the force value for 10% compression was 103N.
[0155] Calculate the content of drug ingredients in the sample according to the weight, use a drug dissolution tester to conduct drug dissolution and degradation experiments on the sample in a simulated ur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Crosslink density | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com