Chicken Infectious Anemia Virus Genetic Engineering Vaccine
A technique for encoding genes and recombining baculoviruses, applied in genetic engineering, viruses, vaccines, etc., can solve problems such as weakened immune response, low expression of VP1, strong virulence, etc., to reduce production costs, improve assembly efficiency, good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] In some embodiments, a method for preparing a chicken infectious anemia virus genetic engineering vaccine specifically includes:
[0070] 1) Prepare a nucleic acid molecule for encoding a chicken infectious anemia virus fusion protein (which can be defined as CIAV-Fu);
[0071] 2) Constructing a recombinant vector, cloning the nucleic acid molecule in step 1) into a shuttle vector to obtain a recombinant shuttle vector containing the target gene;
[0072] 3) Transform the recombinant shuttle vector into DH10Bac bacteria, select the recombinant bacteria, extract the genome and transfect Sf9 cells to obtain recombinant baculovirus;
[0073] 4) Cultivate the Sf9 cells, automatically assemble into virus-like particles (VLP) in the cells, and then recombinantly express and produce a fusion protein (defined as CIAV-VLP);
[0074] 5) Adding the CIAV-VLP into the adjuvant to obtain the vaccine.
[0075] The terminology used in this specification is for the purpose of describi...
Embodiment 1
[0128] Example 1 Construction and Identification of Transfer Vector pF-CIAV-Fu
[0129] 1. CIAV-Fu gene amplification and purification The codon-optimized CIAV-Fu gene (SEQ ID NO: 1) was synthesized in Shanghai Sunny Biotechnology Co., Ltd. and cloned into the pUC17 vector to obtain the pUC-Fu plasmid vector. Using the pUC-Fu plasmid as a template, CIAV-Fu-F and CIAV-Fu-R are used as upstream and downstream primers for PCR amplification (the sequences of CIAV-Fu-F and CIAV-Fu-R are shown in SEQ ID NO: 3 and 4 Shown), the amplification system is shown in Table 1.
[0130] Table 1 CIAV-Fu gene amplification system
[0131]
[0132] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
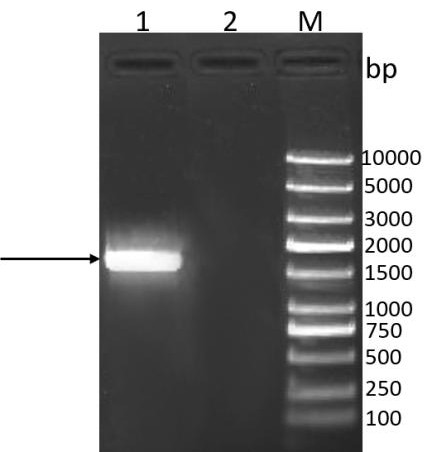
[0133] Perform gel electrophoresis on the PCR product to verify the size of the target gene, such as figure 1 As shown, the target band appea...
Embodiment 2
[0145] Example 2 Construction of recombinant baculovirus genome Bac-CIAV-Fu
[0146]1. Transformation of DH10Bac bacteria Take 1 μl of pF-CIAV-Fu plasmid in Example 1 and add 100 μl of DH10Bac competent cells to mix, ice bath for 30 minutes, heat shock in 42°C water bath for 90 seconds, ice bath for 2 minutes, add 900 μl without Amp LB liquid medium, cultured at 37°C for 5 hours. After 100 μl of bacterial solution was diluted 81 times, 100 μl of diluted bacterial solution was spread on LB solid medium containing gentamicin, kanamycin, tetracycline, X-gal and IPTG, and cultured at 37°C for 48 hours.
[0147] 2. Pick a single colony Use an inoculation needle to pick up large white colonies, then streak on LB solid medium containing gentamicin, kanamycin, tetracycline, X-gal, and IPTG, culture at 37°C for 48 hours, and then pick A single colony was inoculated into LB liquid medium containing gentamicin, kanamycin, and tetracycline for culture, the strains were preserved, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com