Preparation method and application of graphitized carbon with large specific surface area
A large specific surface area, graphitized carbon technology, applied in chemical instruments and methods, graphene, separation methods, etc., can solve problems such as high raw material costs, and achieve a wide range, low surface oxygen content, and rich oxygen-containing functional groups Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
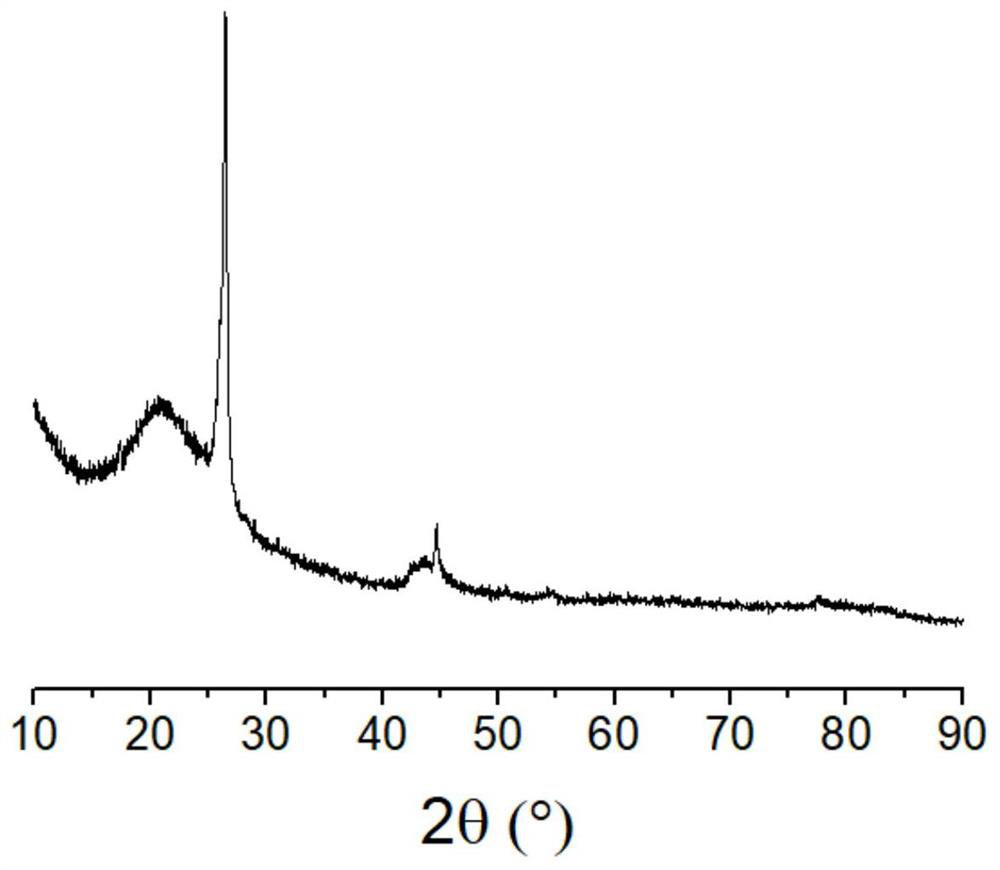
[0045] Dissolve 2 grams of ferric trichloride hexahydrate in 20 ml of water, add 3 grams of activated carbon powder, evaporate the water while stirring, raise the temperature to 150 ° C, keep it for 10 minutes, and cool down to obtain activated carbon loaded with iron. Dissolve 6 grams of potassium hydroxide in 20 milliliters of water, add the above-mentioned activated carbon loaded with iron, evaporate the water while stirring, raise the temperature to 900 ° C in nitrogen, and keep the temperature for 2 hours. After cooling down, put the obtained solid in the Washing in pure water, then soaking in hydrochloric acid solution, finally washing with water until the washing liquid is close to neutral, filtering and drying the solid product to obtain a graphitized carbon product, the BET specific surface area is 1646 square meters per gram, X-ray diffraction The figure shows that it has a graphitized structure (as attached figure 1 Shown), X-ray photoelectron spectroscopy showed th...
Embodiment 2
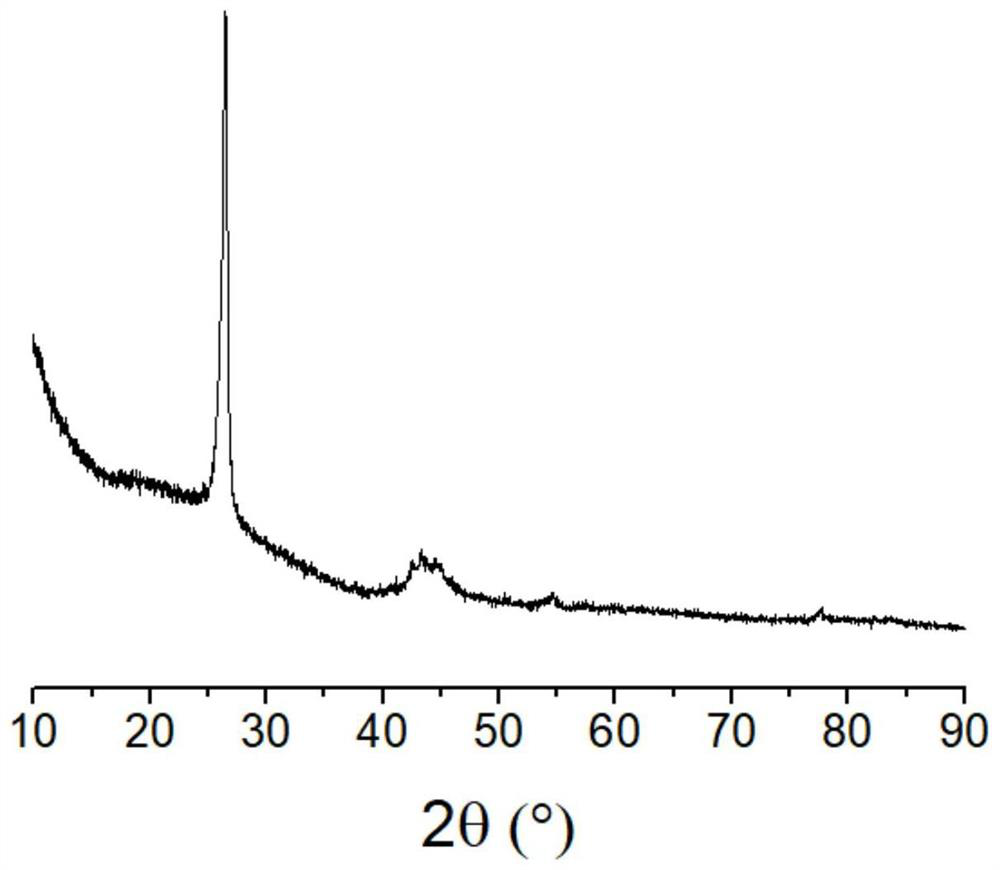
[0050] Dissolve 3 grams of ferric nitrate nonahydrate in 15 milliliters of water, dissolve 1.2 grams of sodium hydroxide in 15 milliliters of water, mix the two to obtain ferric hydroxide precipitate, centrifuge or filter to separate, and add to fresh hydroxide containing bound water Add 1.35 g of acetic acid to the iron, stir until a sol is formed, add water to dilute to 20 ml, and obtain a hydrated iron oxide colloidal solution. Add 3 grams of activated carbon powder to the above colloidal solution, evaporate the water while stirring, raise the temperature to 180° C., keep it for 10 minutes, and cool down to obtain activated carbon loaded with iron. Dissolve 6 grams of potassium hydroxide in 20 milliliters of water, add the above-mentioned activated carbon loaded with iron, evaporate the water while stirring, raise the temperature to 900 ° C in nitrogen, and keep the temperature for 2 hours. After cooling down, put the obtained solid in the Washing in pure water, then soakin...
Embodiment 3
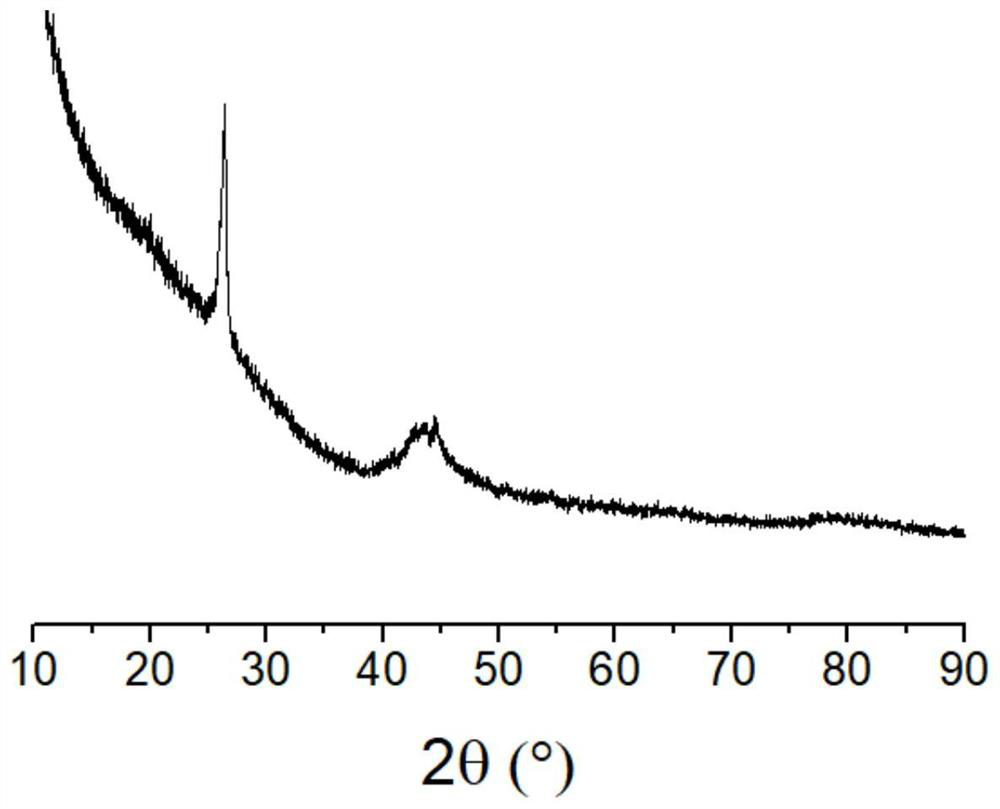
[0053] Dissolve 2.2 g of cobalt nitrate hexahydrate in 20 ml of water, add 3 g of activated carbon powder, evaporate the water while stirring, raise the temperature to 300 ° C in nitrogen, keep it for 10 minutes, and cool down to obtain activated carbon loaded with cobalt. Dissolve 6 grams of potassium hydroxide in 20 milliliters of water, add the above-mentioned activated carbon loaded with cobalt, evaporate the water while stirring, raise the temperature to 900 ° C in nitrogen, and keep the temperature for 2 hours. After cooling down, put the obtained solid in the Washing in pure water, then soaking in hydrochloric acid solution, finally washing with water until the washing liquid is close to neutral, filtering and drying the solid product to obtain a graphitized carbon product, the BET specific surface area is 1617 square meters per gram, X-ray diffraction The figure shows that it has a graphitized structure (as attached image 3 shown).
[0054] If 2.2 grams of cobalt nit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com