Anisodus acutangulus atypical III polyketide synthase and applications thereof
A polynucleotide and amino acid technology, applied in the application field of three-point atypical type III polyketide synthase AaPYKS to prepare 4--3-oxobutyric acid, can solve the problems of large environmental pollution and meet the conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
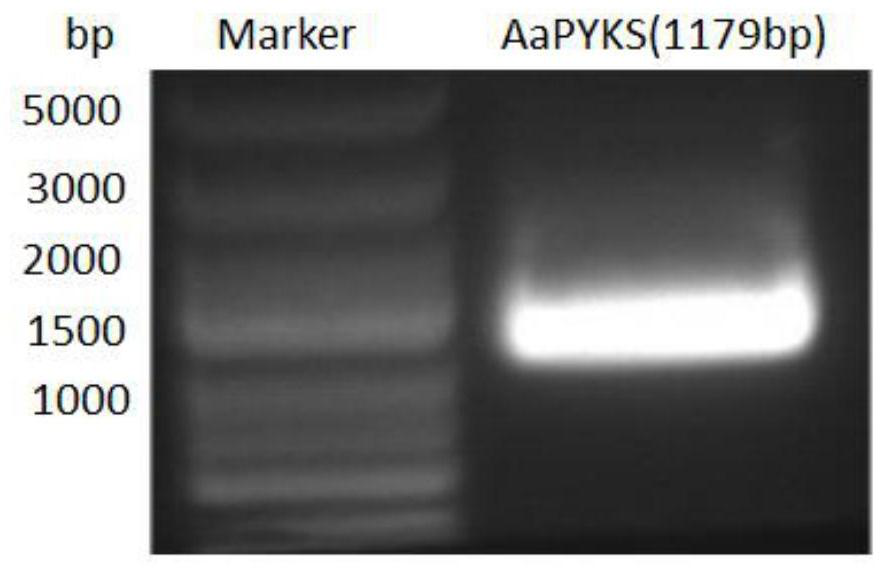
[0064] Example 1 Cloning of three-point-three atypical type III polyketide synthase gene
[0065] 1.1 Extraction and detection of total RNA
[0066]Turn on the ultra-clean bench and ultraviolet lamp, burn the mortar, keys, and scissors with ethanol. Cut off 100mg of three-thirds hairy roots, grind the tissue into powder in liquid nitrogen, and distribute it into Ep tubes. After the liquid nitrogen evaporates, add 1ml Trizol-x-100, shake vigorously immediately or use a pipette to blow 5- 8 times (until there are no lumps), let stand at room temperature for 5 minutes; extract twice with an equal volume of chloroform, and centrifuge at 7500g for 15 minutes; Centrifuge at 10,000 g for 10 min at ℃; add 1 ml of 75% ethanol to the precipitate for washing, and centrifuge at 10,000 g at 4 °C for 10 min; dry the precipitate at room temperature for 10 min and dissolve in 25 μl of DEPC-treated water, and use 1.0% agarose gel electrophoresis to detect the integrity of the RNA. The ratio ...
Embodiment 2 3
[0076] Example 2 Construction of three-point-three atypical type III polyketide synthase expression plasmid
[0077] 2.1 Measure the concentration of the correctly sequenced AaPYKS gene fragment with a Nano-300 instrument, and the concentration is 105 ng / μl.
[0078] 2.2 AaPYKS was double-digested with BamHI and XhoI, and the double-digestion reaction system (50 μl) was as follows: H 2 O, 33 μl; 10x buffer, 5 μl; AaPYKS gene fragment, 10 μl; BamHI, 1 μl; XhoI, 1 μl (restriction enzymes used in the experiment were purchased from Themro Company). Enzymatic digestion was carried out in a water bath at 37°C for 2 hours, and then the digested product was purified using Agarose Gel Fragment Recovery Kit Ver.2.0 from Axygen Company, and the measured concentration was 45ng / μl.
[0079] 2.3 Plasmid pET-24a(+) is an existing vector in our laboratory, and its concentration is 50ng / μl. The pET-24a(+) vector was double-digested with BamHI and XhoI, and the double-digestion reaction syste...
Embodiment 3 3
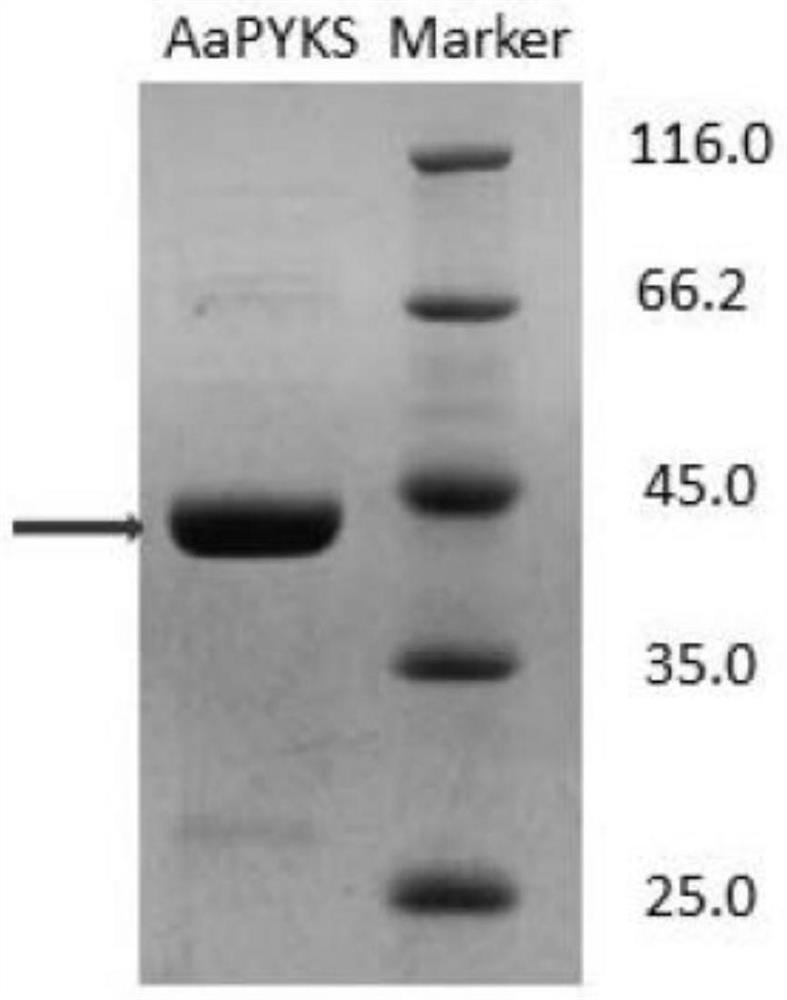
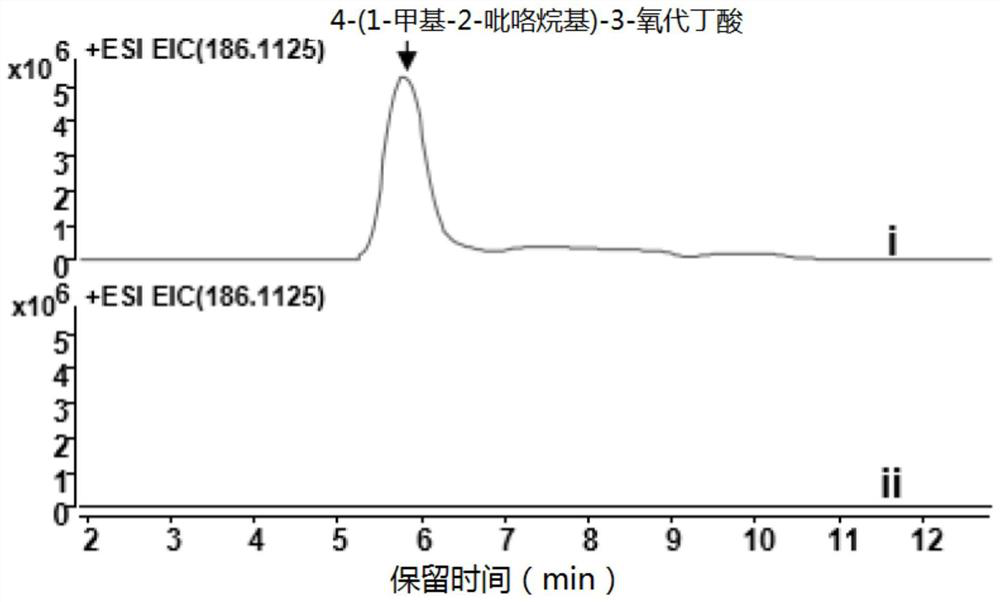
[0084] Example 3 Expression of three-thirds atypical type III polyketide synthase
[0085] 3.1 Take 1 μl of the constructed pET-24a-AaPYKS plasmid (concentration: 120ng / μl), add it to 100 μl of Escherichia coli BL21(DE3) competent cells, put it on ice for 30 minutes, let it stand at 42°C for 90 seconds, and put it on ice for 2 minutes. Add 800 μl of anti-antibody-free LB, and place on a shaker at 37° C. at 220 rpm for 1 hour to recover. Centrifuge at 3000rpm for 1min, remove 800μl supernatant, leave 100μl supernatant to resuspend the bacteria, apply Kan + Resistance plates were placed in a 37°C incubator for 12 hours. Pick a single colony for colony PCR verification, and pick the correct positive clone to 5ml Kan + In the resistant LB test tube, culture at 37°C, 220rpm shaker for 6h. Transfer 5ml cells to 1L Kan + Resistant LB shake flasks were cultured on a shaker at 37°C and 220 rpm. When the OD value of the cells reached 0.6, IPTG with a final concentration of 0.3 mM w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com