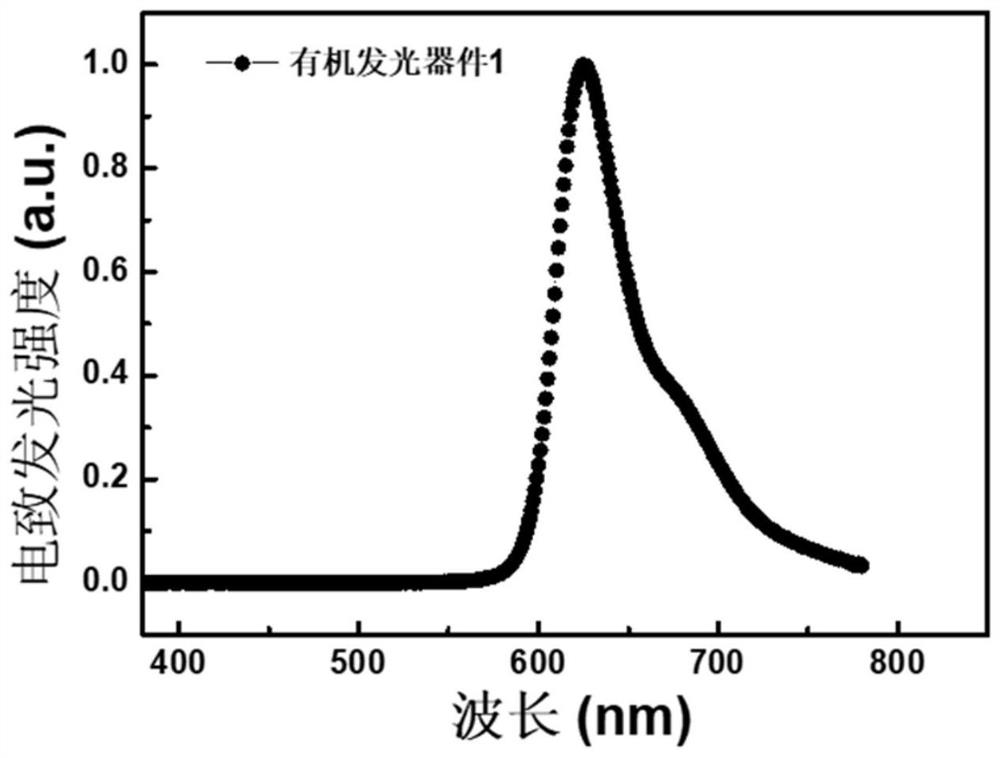
Metatriazine-like compound, electronic device and application
A metatriazine-like compound technology is applied in the field of organic optoelectronic materials to achieve the effects of easy availability of raw materials, enhanced durability, and broad industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
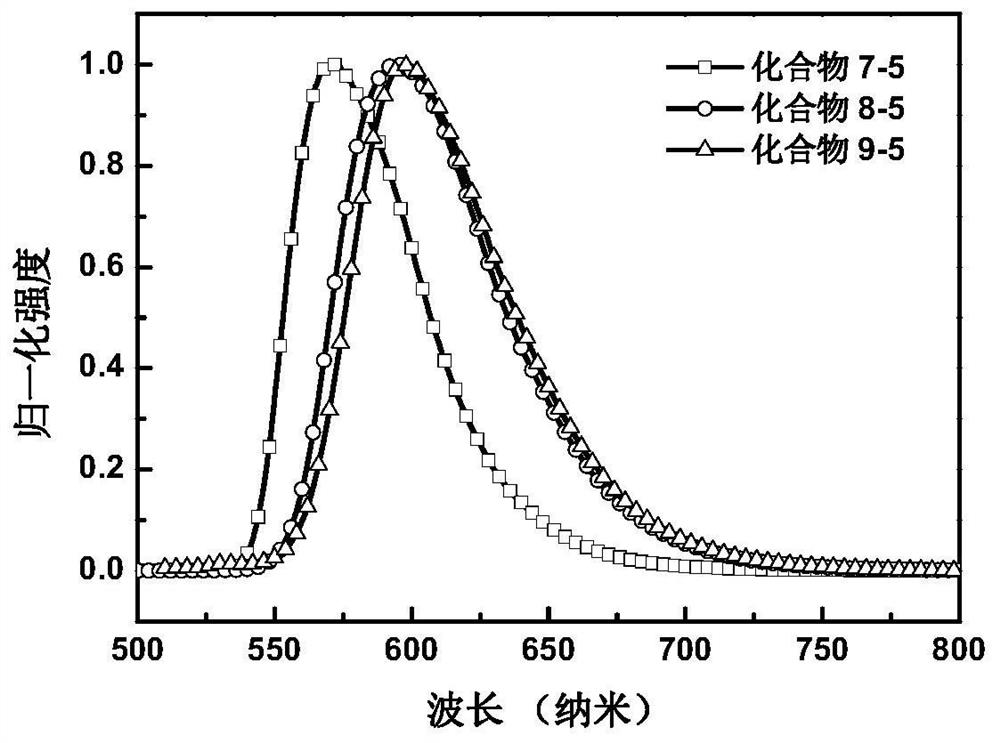
[0120] Embodiment 1: the synthesis of compound 8-5
[0121] (Synthesis of intermediate M1)
[0122] The synthetic route of intermediate M1 is as follows:
[0123]
[0124] P-bromobenzohydrazide (4.3g, 20mmol), ninhydrin (3.6g, 20mmol), ammonium acetate (12.0g, 90mmol) and 120mL acetic acid were successively added into a 250mL single-necked flask, and the reaction was stirred under reflux for 12 hours. After the reaction was complete, the solid was collected by suction filtration and washed with a small amount of absolute ethanol. The crude product was further purified by column chromatography (petroleum ether:dichloromethane=3:1 (V / V)). The solvent was evaporated, and after drying, 3.9 g of a light yellow solid was obtained, with a yield of 58%. MS (EI): m / z: 336.58 [M + ]. Anal.calcdforC 16 h 8 BrN 3 O (%): C 56.83, H 2.38, N 12.43; found: C 56.75, H 2.35, N 12.40.
[0125] (Synthesis of Intermediate M2)
[0126] The synthetic route of intermediate M2 is as follo...
Embodiment 2
[0134] Embodiment 2: the synthesis of compound 9-5
[0135] (Synthesis of compound 9-5)
[0136] The synthetic route of compound 9-5 is as follows:
[0137]
[0138] Under the protection of nitrogen, 2.9 g (8.9 mmol) of 2-bromotriphenylamine and 150 mL of anhydrous tetrahydrofuran were added to a dry and clean 250 mL three-necked flask, and stirred and dissolved at room temperature. The system was cooled to -78°C, and 3.9 mL (2.5 M, 9.8 mmol) of n-butyllithium was added dropwise at this temperature, and stirring was continued at this temperature for 1.5 h after the addition was complete. Subsequently, 4.4 g (8.1 mmol) of intermediate M2 was added in one batch, and the cooling bath was removed after the addition, and the reaction was warmed to room temperature by itself and continued to stir overnight. After the reaction, it was washed with water, dried, and spin-dried to obtain a white solid.
[0139] The above white solid was transferred to a 250mL one-necked bottle equ...
Embodiment 3
[0140] Embodiment 3: the synthesis of compound 7-5
[0141] (Synthesis of compound M3)
[0142] The synthetic route of compound M3 is as follows:
[0143]
[0144] In a 250mL three-neck flask equipped with a reflux condenser and a dropping funnel, add 1g (5.9mmol) iodine element and 100mL glacial acetic acid under nitrogen protection, stir and dissolve, then add about 3.9g (29.6mmol) hypophosphorous acid, and heat up to React at 120°C until the color of the system fades. Then add 11.4g (14.8mmol) M2 at one time, continue to heat and reflux for 4h, cool to room temperature, pour into water to precipitate a large amount of white solid, filter, wash and dry to obtain 6.4g of white crystalline solid, yield 82%. MS (EI): m / z: 526.48 [M + ]. Anal.calcd for C 37 h 24 N 2 (%): C 84.38, H 4.98, N 10.64; found: C 84.30, H 4.95, N 10.63.
[0145] (Synthesis of compound 7-5)
[0146] The synthetic route of compound 7-5 is as follows:
[0147]
[0148] Transfer 7.3g (13.9mm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com