Preparation method of halogenated butyl rubber
A technology of halogenated butyl rubber and butyl rubber, which is applied in the field of rubber, can solve the problems of affecting product quality, high degree of isomerization of brominated products, and increased content of III structure, so as to improve product quality, eliminate the risk of foaming, The effect of increasing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
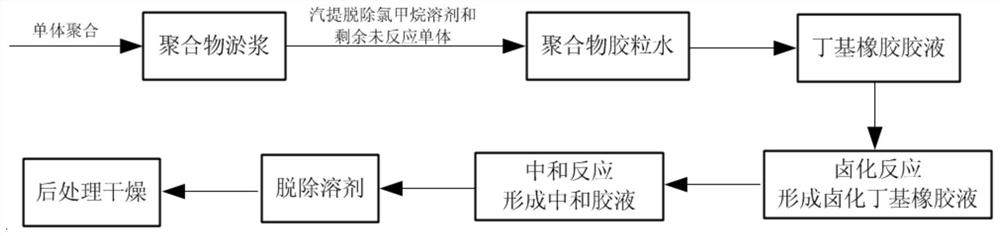
[0038] The invention provides a kind of preparation method of halogenated butyl rubber, comprises the following steps:
[0039]a) sending butyl rubber glue, halogenating agent, oxidizing agent and modifier into a halogenation reactor to carry out halogenation reaction to obtain halogenated butyl rubber liquid;
[0040] b) using an alkali metal hydroxide to neutralize the halogenated butyl rubber solution to obtain a primary neutralization solution;
[0041] c) using calcium chloride to carry out secondary neutralization to the primary neutralization solution to obtain a secondary neutralization solution;
[0042] d) removing the solvent from the secondary neutralization solution and drying to obtain halogenated butyl rubber;
[0043] The improver is selected from one or more of stearic acid and stearate;
[0044] The butyl rubber glue is the butyl rubber glue obtained by hexane stripping.
[0045] In the preparation method provided by the invention, in the halogenation reac...
Embodiment 1
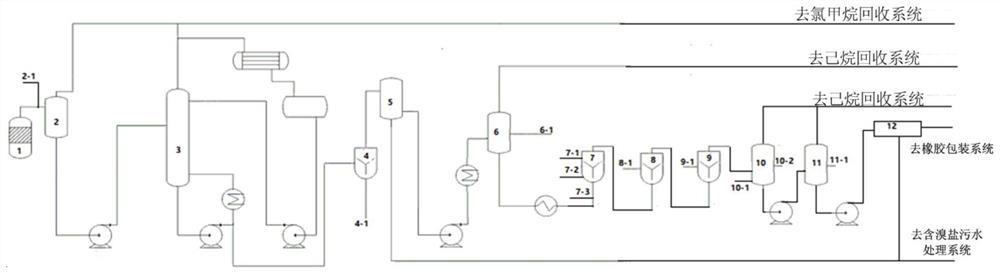
[0115] use figure 2 The device shown was prepared, figure 2 It is a schematic diagram of the production equipment used in the examples of the present invention. Among them, 1-6 are systems for preparing butyl rubber glue, and 7-12 are systems for preparing butyl rubber glue into halogenated butyl rubber. Specifically: 1 is a polymerization reactor, 2 is a flash tank, 3 is a stripper, 4 is the first high-speed mixer, 5 is a buffer device, 6 is a concentration regulator; 7 is a halogenation reactor, 8 and 9 are Neutralization system (8 is the first tank, 9 is the second neutralization tank), 10 is the flash tank, 11 is the stripping tank, and 12 is the drying system.
[0116] 1.1 Preparation of butyl rubber glue:
[0117] With methyl chloride as the medium, AlEtCl 2 and HCl as catalyst (AlEtCl 2 : The mass ratio of HCl is 18:1), and isobutylene and isoprene are monomers (the mass ratio of isobutylene:isoprene is 100:3). AlEtCl 2 Dissolve in methyl chloride to obtain a c...
Embodiment 2
[0136] 1.1 Preparation of butyl rubber glue
[0137] Prepare butyl rubber glue according to the preparation process of Example 1, the difference is that the catalyst deactivator added in flash tank 2 is n-butyl ether, and the n-butyl ether content in glue A is 210ppm; add the first high-speed mixing The amount of water added to device 4 is 3.5wt%. After the heat exchange treatment of the heat exchanger, the temperature of the glue solution is 40°C. The concentration of the final butyl rubber solution is 18.3wt%, the monomer content is 1.9ppm, and the water content is 0.37 wt%, the temperature is 40°C.
[0138] 1.2 Preparation of halogenated butyl rubber
[0139] Utilize gained butyl rubber solution to prepare brominated butyl rubber according to the preparation process of embodiment 1, difference is:
[0140] Bromine sent to the halogenation reactor: the flow rate is 89.5g / h, the weight ratio of bromine to dry glue is 33.0:1000; hydrogen peroxide: the flow rate is 44.38g / h, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com