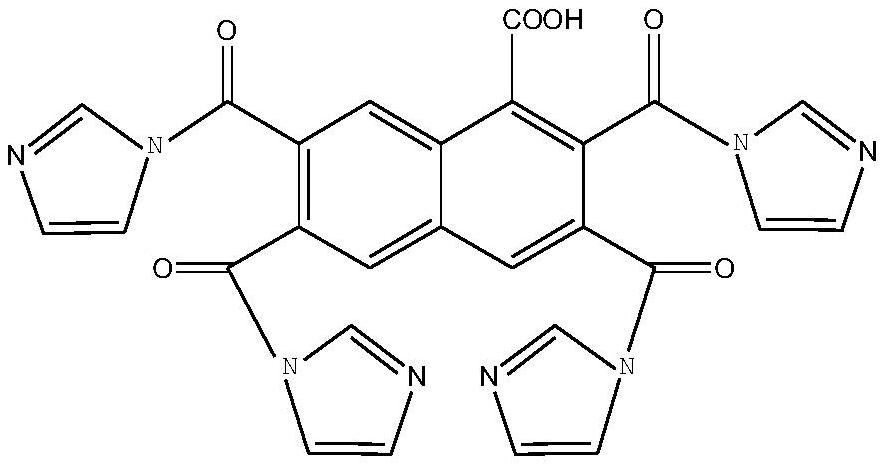
Naphthyl ligand MOF activated carbon composite catalyst and preparation method and application thereof
A technology of catalyst and activated carbon, which is applied in the field of preparing lactide from L-lactic acid, can solve the problems that the reaction conditions require high temperature and high vacuum, the production yield of lactide is reduced, and polylactic acid products cannot be produced. The effect of catalytic activity and stability, controllable molecular weight, and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of organic ligands and synthesis of MOF materials: Take 11.2g of α-naphthoic acid and 5.52g of N,N'-carbonyldiimidazole into 117.162g of dichloromethane solution and stir evenly, and dropwise add 13.12g of three Fluoromethanesulfonic acid was heated at 60°C and stirred for 2h, then 16.69g of cold water was added to the solution, and the mixture was stirred overnight. Product isolation was accomplished by basifying the mixture with sodium hydroxide at a volume ratio of 1:2 for 30 min, and then extracting the mixture twice with chloroform. The organic extract was washed successively with dilute sulfuric acid solution at pH=5 and with water. After the organic phase was distilled, the target ligand was obtained, and anhydrous MgSO 4 Dry and purify by silica gel column chromatography (hexane:ethyl acetate=1:3) multiple times to obtain the target ligand with a yield of 56.3%. Qualitative by NMR analysis: 1 H NMR (D 2 O, 400MHz): δ9.94(s, 1H), 8.93(s, 1H), ...
Embodiment 2
[0045] (1) Preparation of organic ligands and synthesis of MOF materials: Take 23.14g of α-naphthoic acid and 8.72g of N,N'-carbonyldiimidazole into 187.62g of dichloromethane solution and stir evenly, and dropwise add 21g of trifluoro Methanesulfonic acid was heated at 60°C and stirred for 2h, then 26.8g of cold water was added to the solution, and the mixture was stirred overnight. Product isolation was accomplished by basifying the mixture with sodium hydroxide at a volume ratio of 1:1 for 30 min, and then extracting the mixture twice with chloroform. The organic extract was washed three times successively with dilute sulfuric acid solution of pH=6 and water. After the organic phase was distilled, the target ligand was obtained, and anhydrous MgSO 4 Dry and purify by silica gel column chromatography (hexane:ethyl acetate=1:3) multiple times to obtain the target ligand with a yield of 57.2%; take 5.48g of the ligand and 3.6g and 2.1g of the active metal component nickel nit...
Embodiment 3
[0048] (1) Preparation of organic ligands and synthesis of MOF materials: Take 18.217g of α-naphthoic acid and 7.657g of N,N'-carbonyldiimidazole into 133.84g of dichloromethane solution and stir evenly, and dropwise add 16.057g of three Fluoromethanesulfonic acid was heated at 60°C and stirred for 2h, then 22.257g of cold water was added to the solution, and the mixture was stirred overnight. Product isolation was accomplished by basifying the mixture with sodium hydroxide at a volume ratio of 1:1 for 30 min, and then extracting the mixture twice with chloroform. The organic extract was washed three times successively with dilute sulfuric acid solution of pH=6 and water. After the organic phase was distilled, the target ligand was obtained, and anhydrous MgSO 4 Dry and purify by silica gel column chromatography (hexane:ethyl acetate=1:3) multiple times to obtain the target ligand with a yield of 56.2%. Take 5.48g of ligand, 3.19g of active metal components aluminum nitrate,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distribution coefficient | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com