Magnetic material loaded N-heterocyclic carbene copper catalyst as well as preparation method and application thereof
A nitrogen-heterocyclic carbene and magnetic material technology, which is applied in catalytic reactions, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as increasing the difficulty of product separation, increasing production costs, and destroying ecosystems. Industrial application prospects, cost saving, and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
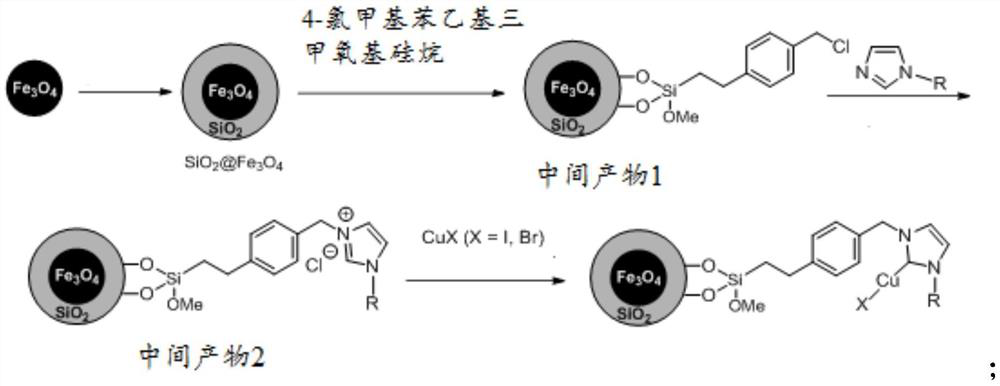
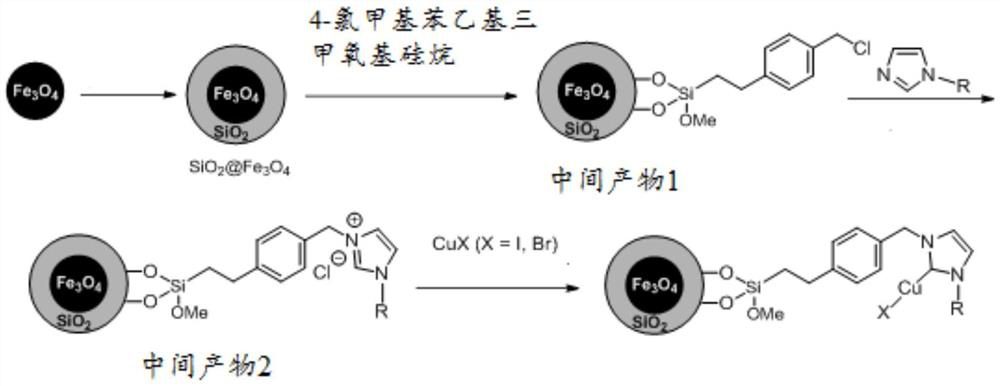
[0032] The invention provides a method for preparing a magnetic material-supported nitrogen heterocyclic carbene copper catalyst, comprising the following steps:
[0033] S1, with Fe 3 o 4 and tetraethyl orthosilicate as raw materials to prepare SiO under alkaline conditions 2 Coated Fe 3 o 4 magnetic nanoparticles;
[0034] S2, take 4-chloromethylphenethyltrimethoxysilane as raw material, in the SiO 2 Coated Fe 3 o 4 The surface of magnetic nanoparticles was functionalized with benzyl chloride to obtain intermediate product 1;
[0035] S3. Under a protective gas atmosphere, using N-substituted imidazole as a raw material, carry out imidazolium salt grafting on the surface of the intermediate product 1 to obtain an intermediate product 2;
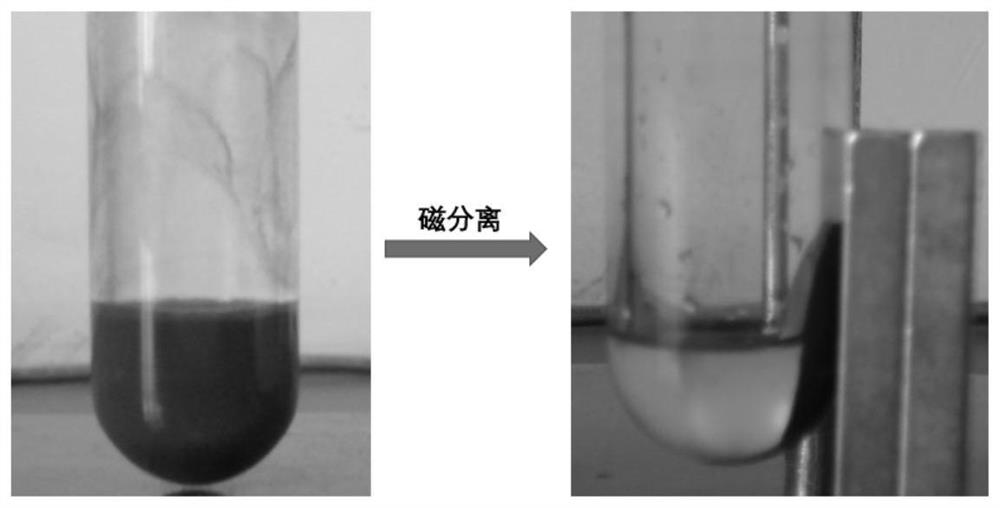
[0036] S4. Under a protective gas atmosphere, disperse cuprous halide, sodium tert-butoxide or potassium tert-butoxide, and the intermediate product 2 in a solvent, stir and react at room temperature, and then use a magnet to adsorb...
Embodiment 1
[0042] A preparation method of a magnetic material loaded nitrogen heterocyclic carbene copper catalyst, comprising the following steps:
[0043] (1) Preparation of silica-coated magnetic nanoparticles
[0044] Commercial Fe 3 o 4 Particles (average diameter 20nm, 0.25g) were sonicated with 0.1M dilute hydrochloric acid for 5 minutes, separated and washed with a magnet, added to a 1000mL reaction bottle, and then NH 3 ·H 2 O (2.0 mL), deionized water (100.0 mL), ethanol (400.0 mL), and the mixed solution was sonicated for about 1 h. After the solid is fully dispersed, slowly add tetraethyl orthosilicate (TEOS, 0.4g) dropwise into the suspension, and continue to stir the suspension at room temperature for 12 hours. Wash with water until neutral, then wash with ethanol and ether three times respectively, and dry in vacuum to finally obtain 0.31 g of the product.
[0045] (2) Functionalization of the surface of silica-coated magnetic nanoparticles with benzyl chloride
[00...
Embodiment 2
[0052] A preparation method of a magnetic material loaded nitrogen heterocyclic carbene copper catalyst, comprising the following steps:
[0053] (1) Preparation of silica-coated magnetic nanoparticles
[0054] Commercial Fe 3 o 4 Particles (average diameter 20nm, 0.5g) were sonicated with 0.1M dilute hydrochloric acid for 15 minutes, separated and washed with a magnet, added to a 2000mL reaction bottle, and then NH 3 ·H 2 O (4.0 mL), deionized water (200.0 mL), ethanol (800.0 mL), and the mixed solution was sonicated for about 2 h. After the solids were fully dispersed, tetraethyl orthosilicate (TEOS, 0.6 g) was slowly added dropwise to the suspension, and the suspension was stirred at room temperature for 12 hours, and the finally obtained product was washed with deionized water until neutral, and then Wash with ethanol and ether three times respectively, and dry in vacuum to finally obtain 0.63 g of the product.
[0055] (2) Functionalization of the surface of silica-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com