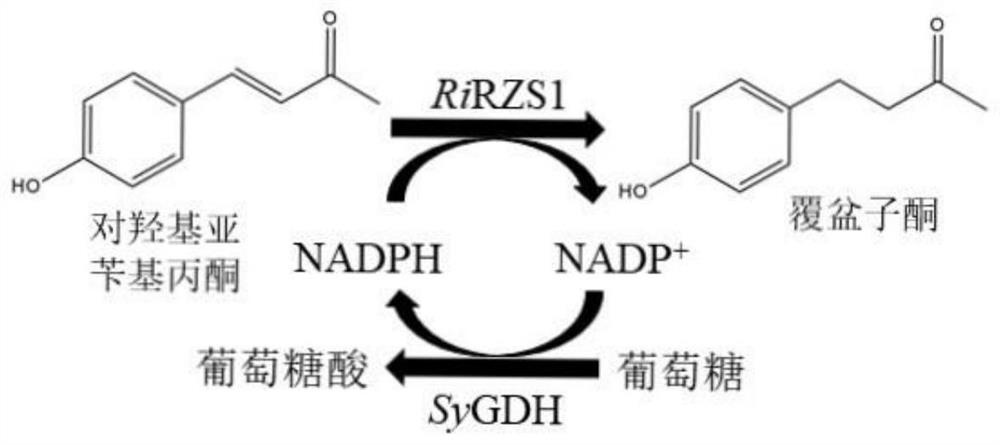
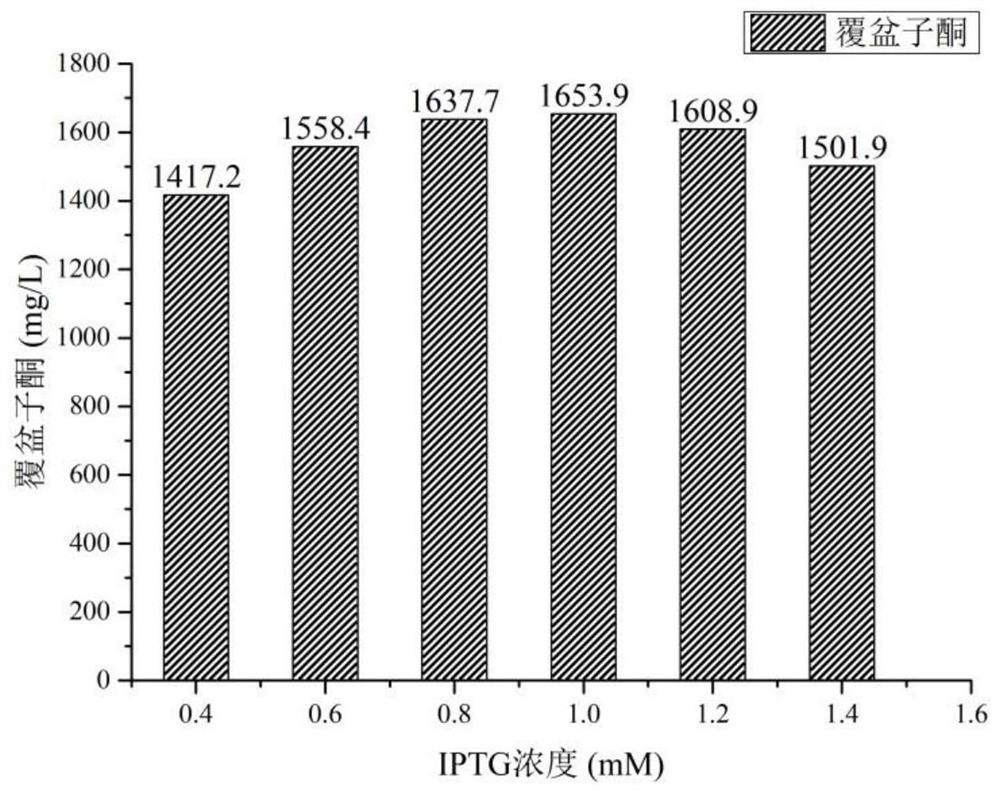
Method for preparing raspberry ketone by whole-cell transformation
A technology of whole cell transformation and raspberry ketone, applied in the field of genetic engineering, can solve the problems of complex preparation method, long fermentation time, equipment corrosion, etc., and achieve the effects of environmental protection, high transformation efficiency and low energy consumption in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]Example 1: Construction of a recombinant plasmid containing the glucose dehydrogenase gene sygdh
[0059]Specific steps are as follows:
[0060]The codon-optimized nucleotide sequence of the glucose dehydrogenase gene sygdh shown in SEQ ID NO. 2 was used as a template, and sygdh-F and sygdh-R were used as primers for PCR amplification; PCR reaction conditions were: 90 -95°C pre-denaturation for 3 to 5 minutes, 94°C denaturation for 30 to 45 seconds, 55°C annealing for 30 to 45 seconds, 70 to 72°C extension for 1 minute, 30 cycles, 70 to 72°C fully extended for 5 to 10 minutes.
[0061]After the PCR reaction, the PCR product was detected by agarose gel electrophoresis, and the gel was cut to recover the glucose dehydrogenase gene sygdh. The primers are:
[0062]sygdh-F: GGATCCATGACCGAACAGAAAG (SEQ ID NO. 3)
[0063]sygdh-R: AAGCTTGCGGGCCGCTTACTGC (SEQ ID NO. 4)
[0064]Connect the glucose dehydrogenase gene sygdh obtained in the above steps to the vectors pETDuet-1, pACYCDuet-1, pCDFDuet-1 and p...
Embodiment 2
[0065]Example 2: Construction of a recombinant plasmid containing the glucose dehydrogenase gene sygdh and the benzylacetone reductase gene rirzs1
[0066]Specific steps are as follows:
[0067]The benzylacetone reductase gene rirzs1 with the codon-optimized nucleotide sequence shown in SEQ ID NO. 1 was used as a template, and rirzs1-F, rirzs1-R were used as primers for PCR amplification; the PCR reaction conditions were: 90-95°C pre-denaturation for 3 to 5 minutes, 94°C denaturation for 30 to 45 seconds, 55°C annealing for 30 to 45 seconds, 70 to 72°C extension for 1 minute, 30 cycles, 70 to 72°C fully extended for 5 to 10 minutes.
[0068]After the PCR reaction, the PCR product was detected by agarose gel electrophoresis, and the gel was cut to recover the benzylacetone reductase gene rirzs1. The primers are:
[0069]rirzs1-F: AGATCTATGGCCAGCGGC (SEQ ID NO.5)
[0070]rirzs1-R: GGTACCTTATTCACGGCTAACCA (SEQ ID NO.6)
[0071]The benzylacetone reductase gene rirzs1 obtained in the above steps is ligate...
Embodiment 3
[0072]Example 3: Construction of recombinant E. coli co-expressing benzylacetone reductase and glucose dehydrogenase
[0073]Specific steps are as follows:
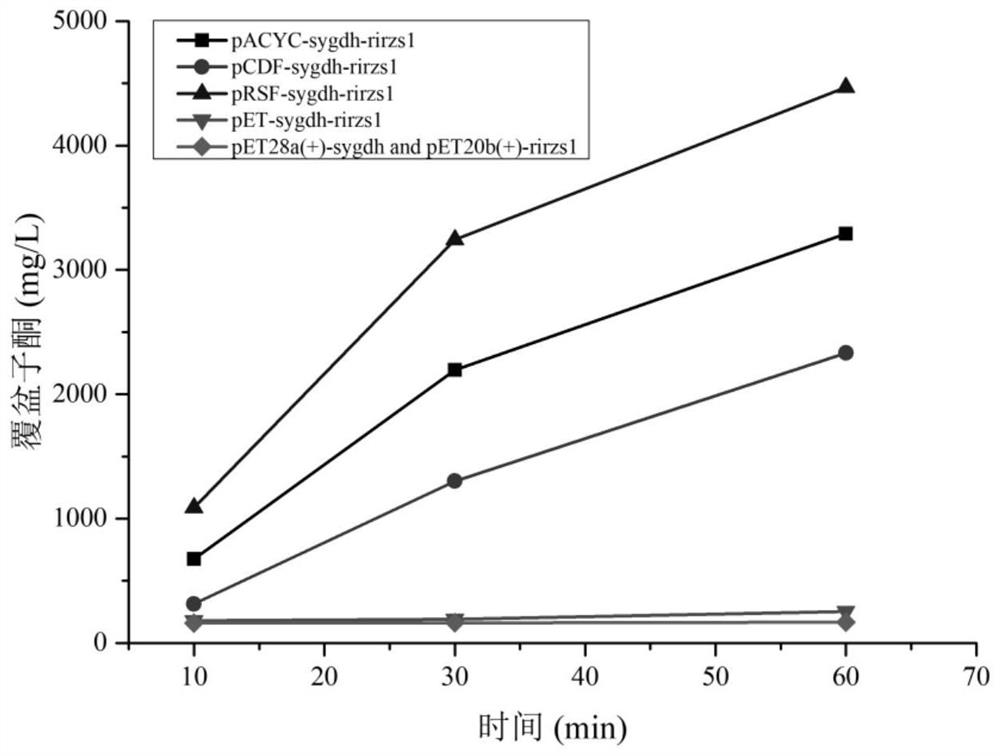
[0074](1) The recombinant plasmids pET-sygdh-rirzs1, pACYC-sygdh-rirzs1, pCDF-sygdh-rirzs1 and pRSF-sygdh-rirzs1 constructed in Example 2 were respectively transferred into E. coli BL21 (DE3) competent cells to obtain Single plasmid recombinant engineering strains BL21 / pET-sygdh-rirzs1, BL21 / pACYC-sygdh-rirzs1, BL21 / pCDF-sygdh-rirzs1 and BL21 / pRSF-sygdh-rirzs1.
[0075](2) Construction of recombinant engineering strains containing dual plasmid expression vectors pET20b(+)-rirzs1 and pET28a(+)-sygdh
[0076]Reduce the glucose dehydrogenase gene sygdh with the codon-optimized nucleotide sequence as shown in SEQ ID NO.2 and the codon-optimized nucleotide sequence as the benzylacetone shown in SEQ ID NO.1 The enzyme gene rirzs1 was used as a template, and sygdh-F-Nco I, sygdh-R-Xho I, rirzs1-F-Nde I, and rirzs1-R-Kpn I were used as primers for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com