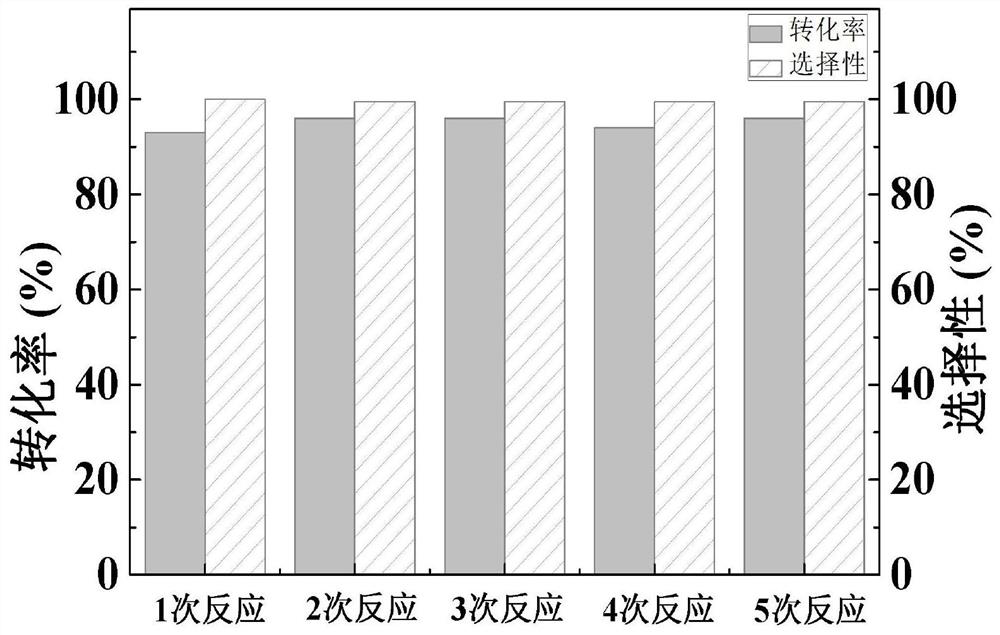
A preparation method of nitrogen-doped carbon nanomaterial and its application in hydrogenation reaction of nitrobenzene
A nanomaterial, nitrogen-doped carbon technology, applied in the preparation of organic compounds, the preparation of amino compounds, chemical instruments and methods, etc., can solve the problems of corrosion equipment and storage hazards, high production costs, and high production equipment and process control requirements. problem, to achieve the effect of low raw material price, short time consumption, and improved catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The embodiment of the present invention discloses a method for preparing nitrogen-doped carbon nanomaterials derived from nitrogen-rich covalent organic framework materials. : 1.5 was dissolved in 36mL dimethyl sulfoxide solution, the solution mixture was transferred to the reaction kettle at 165°C for 72h, cooled naturally, the product was filtered with suction, washed several times with absolute ethanol, acetone, tetrahydrofuran, and dichloromethane successively, and dried to obtain The white powder is then subjected to high-temperature carbonization in a tube furnace to obtain carbon nanomaterial CC-X (X represents the carbonization temperature).
[0039] On the basis of the above-mentioned CC-X catalyst provided by the present invention, the applicant has carried out research on using CC-X catalysts with different carbonization temperatures to catalyze the hydrogenation reaction of nitrobenzene. The steps of the catalytic reaction are as follows:
[0040] Add cataly...
Embodiment 5
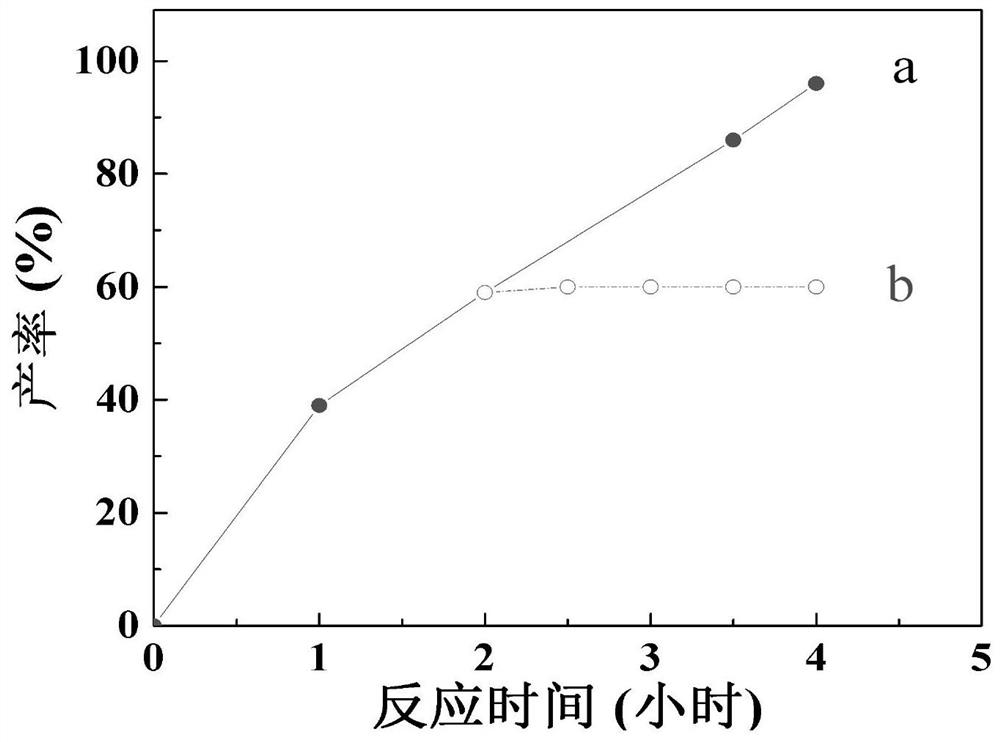
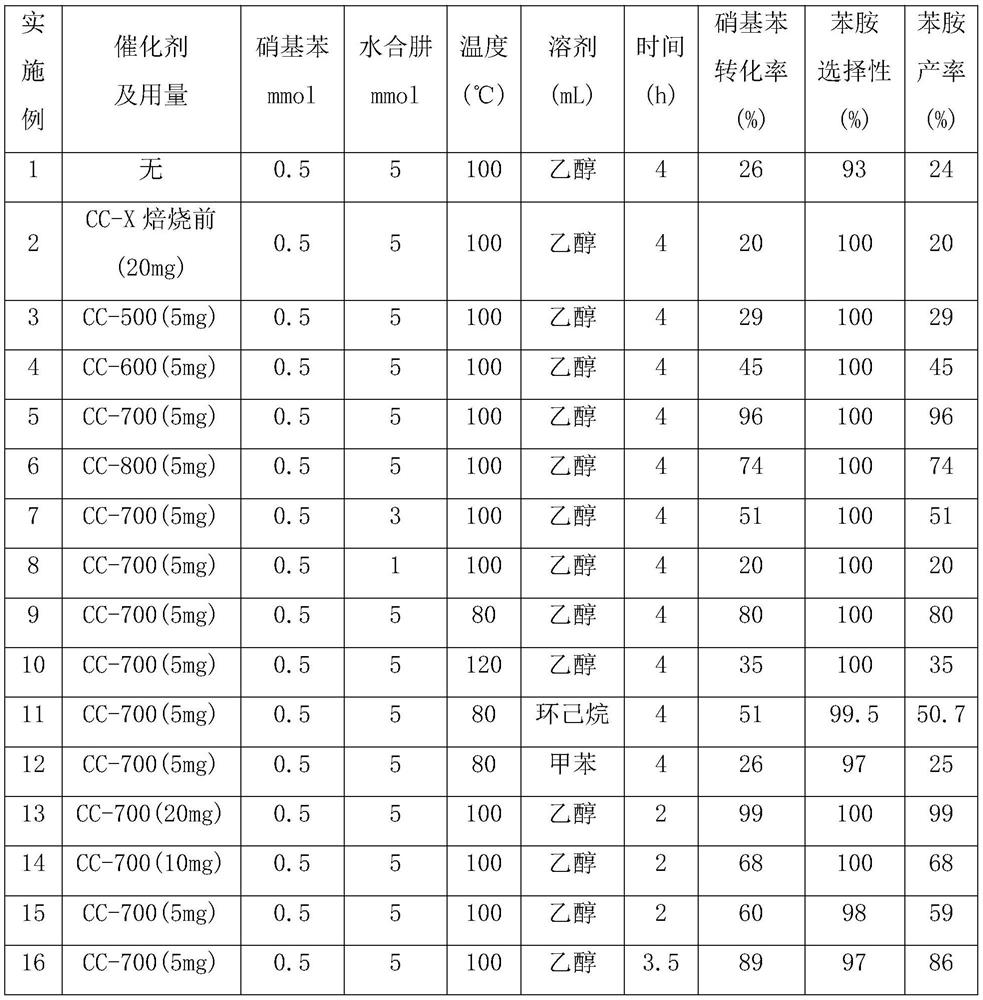
[0049] All use CC-700 as catalyst in embodiment 5,9,10, the consumption that adds hydrazine hydrate is also the same, but the temperature of reaction is different, other catalytic reaction conditions are all the same. It can be seen from the experimental data that the conversion of nitrobenzene is beneficial to the increase of the reaction temperature. In Example 5, the reaction temperature is 100° C., and the conversion rate of nitrobenzene is 96%. But the reaction temperature is too high. In Example 10, the conversion rate of nitrobenzene drops to 35%, which may be related to the evaporation of solvent ethanol and reducing agent hydrazine hydrate.
[0050] In Examples 9, 11, and 12, CC-700 was used as a catalyst, and the same amount of hydrazine hydrate was added as a reducing agent. The reaction temperature was 80°C, but the solvents added were different. After 4 hours of reaction, ethanol was used as a solvent for the reaction of nitro Both the conversion of benzene and th...
Embodiment 13
[0051] In Examples 13, 14, and 15, except that the amount of catalyst CC-700 added is different, other catalytic reaction conditions are the same. It can be seen from the experimental data that the amount of catalyst CC-700 added in Example 13 is the largest, the yield of aniline is also the highest, the catalytic effect is good, and the utilization rate of the catalyst is 15mmol g -1 h -1 . In Example 15, the amount of catalyst CC-700 added is the least, and the yield of aniline is also the lowest, but the utilization rate of the catalyst is 30mmol·g -1 h -1 , is the highest among the three examples.
[0052] In Examples 5, 15, and 16, the catalytic reaction conditions are the same, only the reaction time is different. It can be seen that the yield of aniline has been significantly improved with the increase of the reaction time.
[0053] in addition, figure 1 It is a line graph showing the change of aniline output with reaction time when catalyzing the hydrogenation rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com