Novel catalytic process for arylamine aromatic hydrocarbon alkylation
A technology for the alkylation of arylamine aromatic hydrocarbons, which is applied in the field of new catalytic technology for alkylation of aromatic amine aromatic hydrocarbons, can solve the problems of poor heat transfer performance, achieve high service life, high activity service life, and improve the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
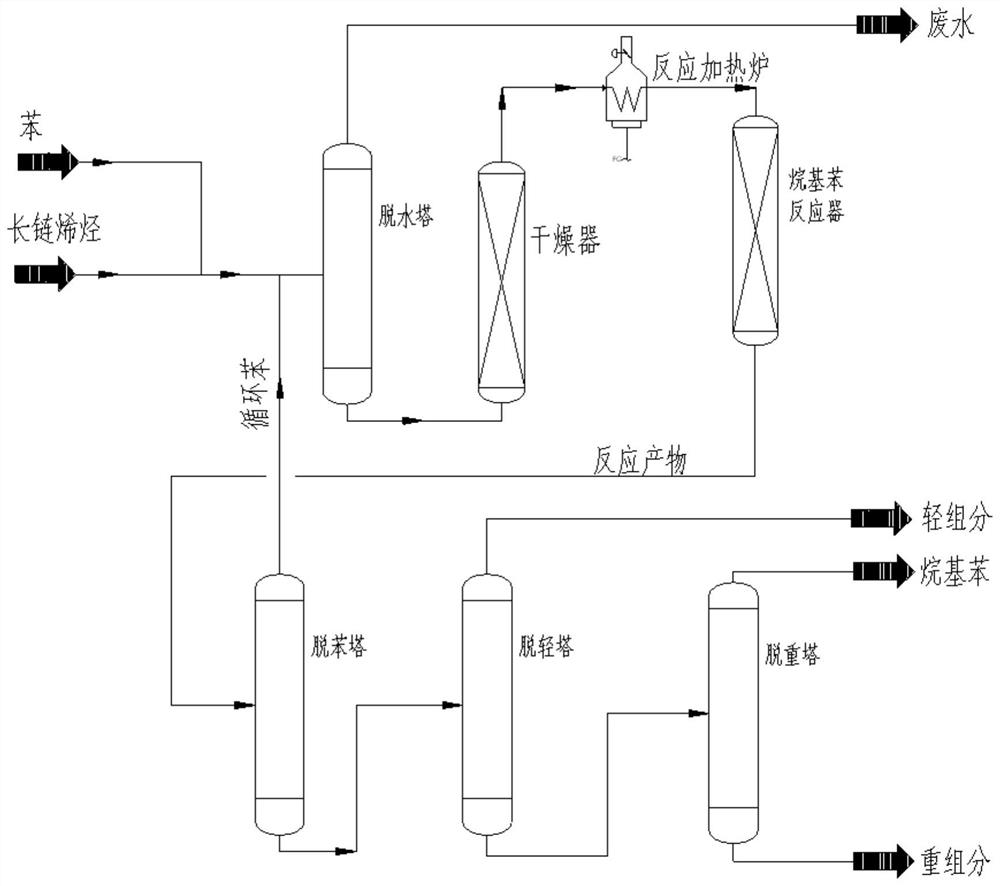
[0043] see Figure 1-2 , a novel catalytic process for the alkylation of arylamine aromatic hydrocarbons, comprising the following steps:
[0044] S1. Add benzene and long-chain olefins into the dehydration tower in proportion, and enter the dryer for drying after reflux dehydration;
[0045] S2. The raw material after drying treatment is heated to 130° C. in a reaction heating furnace, and fully reacted in the alkylbenzene reactor under the catalysis of a new catalyst to obtain a reaction product;
[0046] S3, the reaction product enters the atmospheric pressure distillation in the debenzene tower to remove the unreacted benzene, and then recycles it;
[0047] S4. The reaction product after benzene removal is sequentially subjected to vacuum distillation in a light removal tower and a weight removal tower to remove light components and heavy components in the reaction product to obtain pure alkylbenzene.
[0048] The molar ratio of benzene and long-chain olefins in step S1 ...
Embodiment 2
[0058] see Figure 1-2 , a novel catalytic process for the alkylation of arylamine aromatic hydrocarbons, comprising the following steps:
[0059] S1. Add benzene and long-chain olefins into the dehydration tower in proportion, and enter the dryer for drying after reflux dehydration;
[0060] S2. The raw material after drying treatment is heated to 150° C. in a reaction heating furnace, and fully reacted in the alkylbenzene reactor under the catalysis of a new catalyst to obtain a reaction product;
[0061] S3, the reaction product enters the atmospheric pressure distillation in the debenzene tower to remove the unreacted benzene, and then recycles it;
[0062] S4. The reaction product after benzene removal is sequentially subjected to vacuum distillation in a light removal tower and a weight removal tower to remove light components and heavy components in the reaction product to obtain pure alkylbenzene.
[0063] The molar ratio of benzene and long-chain olefins in step S1 ...
Embodiment 3
[0069] see Figure 1-2 , a novel catalytic process for the alkylation of arylamine aromatic hydrocarbons, comprising the following steps:
[0070] S1. Add benzene and long-chain olefins into the dehydration tower in proportion, and enter the dryer for drying after reflux dehydration;
[0071] S2. The raw material after drying treatment is heated to 180°C in a reaction heating furnace, and fully reacted in the alkylbenzene reactor under the catalysis of a new catalyst to obtain a reaction product;
[0072] S3, the reaction product enters the atmospheric pressure distillation in the debenzene tower to remove the unreacted benzene, and then recycles it;
[0073] S4. The reaction product after benzene removal is sequentially subjected to vacuum distillation in a light removal tower and a weight removal tower to remove light components and heavy components in the reaction product to obtain pure alkylbenzene.
[0074] The molar ratio of benzene and long-chain olefins in step S1 is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com