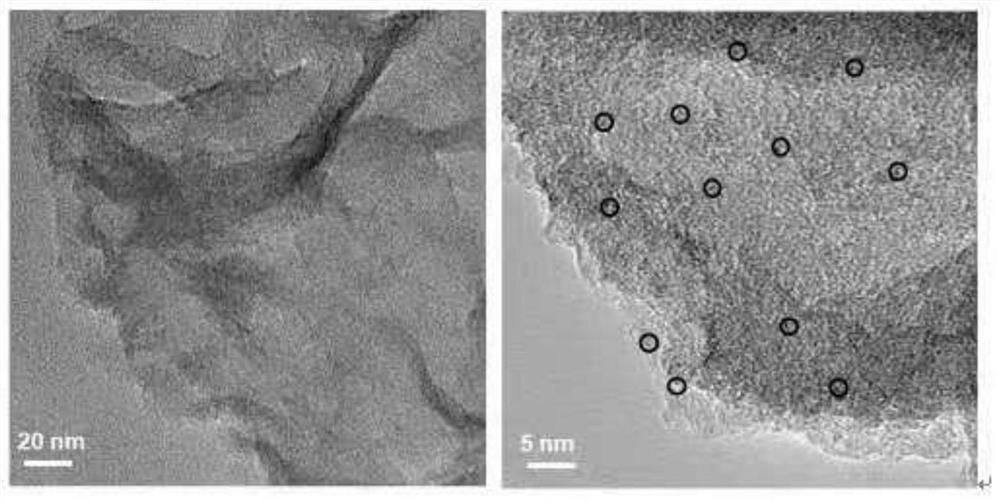
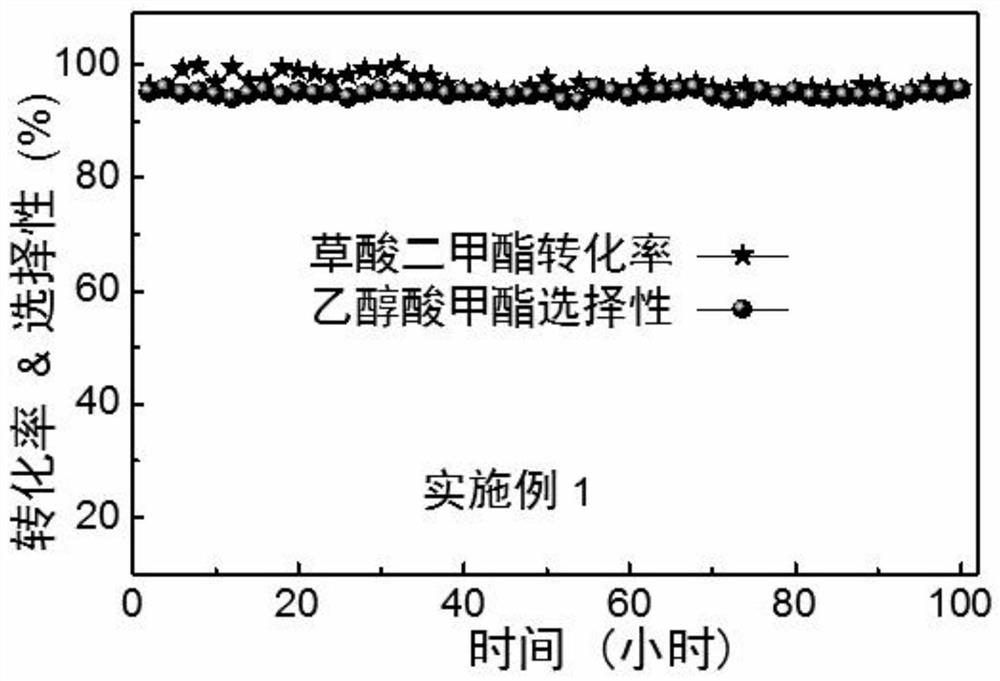
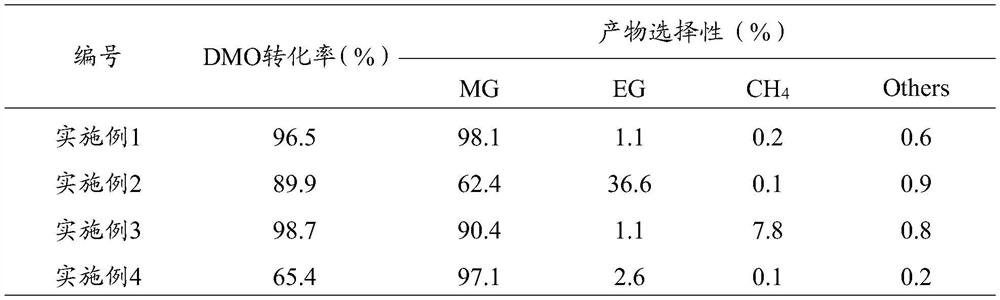
Modified boron nitride supported metal catalyst and preparation method and application thereof
A technology for supporting metals and boron nitride, which is applied in physical/chemical process catalysts, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve problems such as large amount of metal consumption, high processing costs, environmental protection pressure, and easy agglomeration of metal sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] The present invention also provides a preparation method of the modified boron nitride-supported metal catalyst described in the above technical scheme, comprising the following steps:
[0072] 1, the modified boron nitride carrier is dispersed in a solvent to obtain a colloidal solution;
[0073] II. Under stirring conditions, the colloidal solution obtained in the step I is mixed with a metal salt solution containing active component elements and then adsorbed to obtain a precursor suspension;
[0074] III. Centrifuging and drying the precursor suspension obtained in step II in turn to obtain a precipitate;
[0075] IV. Reductive heat treatment is performed on the precipitate obtained in the step III to obtain a modified boron nitride-supported metal catalyst.
[0076] In the invention, the modified boron nitride carrier is dispersed in a solvent to obtain a colloidal solution.
[0077] In the present invention, the solvent is preferably water, ethanol, acetone, eth...
Embodiment 1
[0100] The preparation method of modified boron nitride supported metal catalyst comprises the following steps:
[0101] 1. Disperse 200mg of the modified boron nitride carrier in 50mL of water, after ultrasonication at 40W for 2h, stirring at 1000r / min to form a colloidal solution with a concentration of 4mg / mL;
[0102] II. Dissolve 250mg hexaammine ruthenium chloride in water, and make it into a 10mg / mL aqueous solution of hexaammine ruthenium chloride in a 25mL volumetric flask. Under the stirring condition of 1000r / min at room temperature, The volume ratio of the hexaammine ruthenium chloride aqueous solution to the colloidal solution is 1:100, and the above-mentioned hexaammine ruthenium chloride aqueous solution is added dropwise to the colloidal solution obtained in the step 1, and the stirring is continued for 18h to obtain a precursor suspension;
[0103] III. Centrifuge the precursor suspension obtained in step II for 10 minutes at 10,000 r / min to collect the precip...
Embodiment 2
[0110] The preparation method of modified boron nitride supported metal catalyst comprises the following steps:
[0111] 1. Disperse 200mg of the modified boron nitride carrier in 50mL of water, after ultrasonication at 40W for 2h, stirring at 1000r / min to form a colloidal solution with a concentration of 4mg / mL;
[0112] II. Dissolve 400mg of copper acetylacetonate in water, and make it into a 16mg / mL aqueous solution of copper acetylacetonate at a constant volume in a 25mL volumetric flask. The volume ratio of the above-mentioned copper acetylacetonate solution is 1:100, and 0.5mL of the above-mentioned copper acetylacetonate aqueous solution is added dropwise to the colloidal solution obtained in the step 1, and the stirring is continued for 18h to obtain a precursor suspension;
[0113] III. Centrifuge the precursor suspension obtained in step II for 10 minutes at 10,000 r / min to collect the precipitate, and freeze-dry it for 18 hours to obtain the precipitate;
[0114] I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com