Method for preparing salicylate through green synthesis process
A technology of salicylates and methyl salicylate, which is applied in the field of green synthesis of salicylates, can solve the problems of treatment, large amounts of waste water and waste salt, achieve smooth reaction, improve quality and yield rate, reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
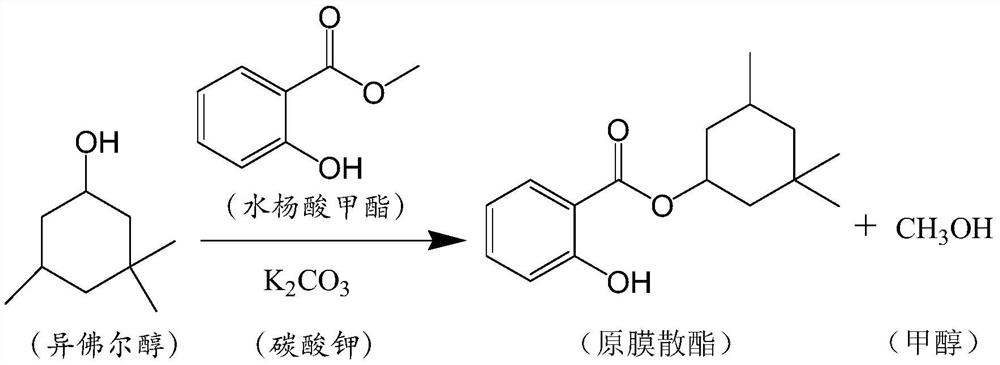
[0044] According to the present invention, one aspect is to provide a preparation method of salicylate green synthesis process, said method comprising the following steps:
[0045] Step 1, mixing salicylate compounds and isophorol, optionally adding a catalyst.
[0046] In step 1, the salicylate compound is a salicylate, preferably an alkyl salicylate, preferably selected from methyl salicylate, ethyl salicylate, isopropyl salicylate, One or more of isobutyl salicylate and isoamyl salicylate.
[0047] Preferably, the salicylate compound is methyl salicylate.
[0048] In the present invention, methyl salicylate has the advantages of simple structure among salicylate compounds, small steric hindrance, low toxicity, environmental friendliness, simple and mature synthesis process, and low requirements for equipment. More importantly, under the action of a catalyst, methyl salicylate is easily decomposed under heating conditions, and the products are salicylic acid and methanol. ...
Embodiment 1
[0092] In a 1000ml four-necked flask with a rectification tower, 152g of methyl salicylate, 426g of isophorol, and 14g of potassium carbonate were fed.
[0093] Turn on the water circulation vacuum pump, adjust the vacuum pressure to -0.085MPa, start stirring and raise the temperature to 115-120°C; continue to produce methanol, when the methanol production slows down, add 100g of toluene, slowly increase the vacuum degree to -0.095MPa, the large reflux is small The final small amount of methanol is taken out until the reaction is completed, the total reaction time is 3 hours, the conversion rate is greater than 99%, and the selectivity is greater than 99%.
[0094] After the reaction, the temperature was lowered to 70-80°C, potassium carbonate was removed by filtration, and the recovered potassium carbonate was directly used in the transesterification step, and the filtrate was transferred into a four-neck flask.
[0095] Add a small amount of acetic acid to adjust the pH of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com