Application of pyrene-1-carboxaldehyde and detection method of biological small molecules
A technology of small biological molecules and detection methods, which is applied in the detection of small biological molecules and the application field of 1-pyrene formaldehyde, can solve problems such as difficult simultaneous qualitative and quantitative analysis, detection structure influence, complex system, etc. High chemical efficiency, low purchase cost, and highly sensitive quantitative detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
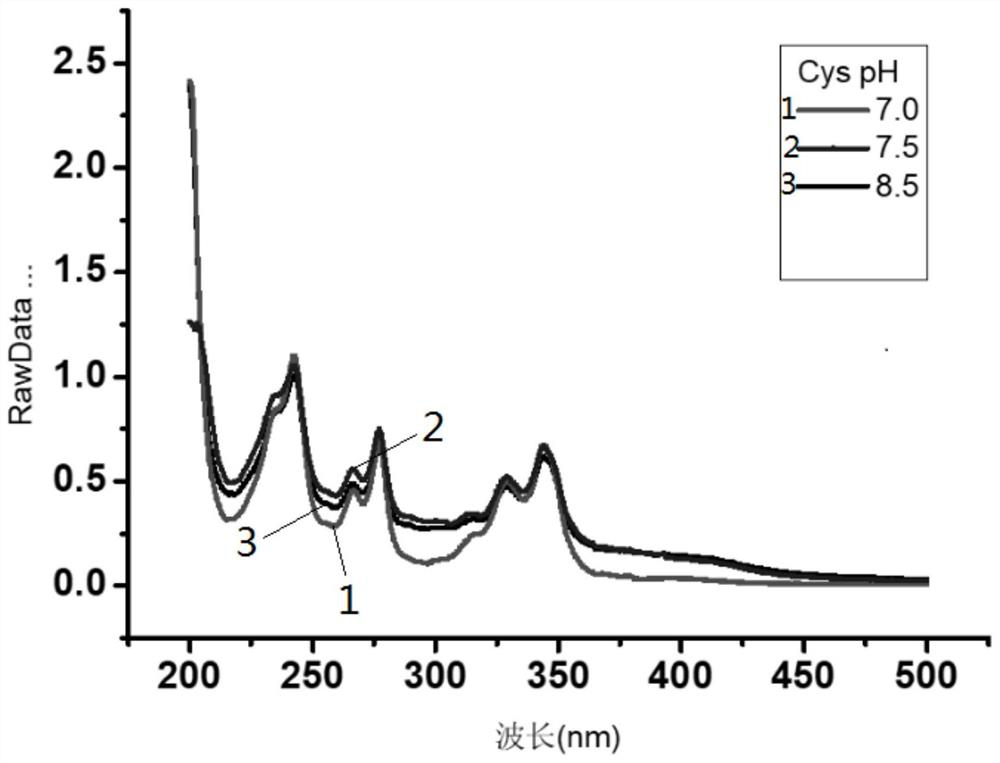
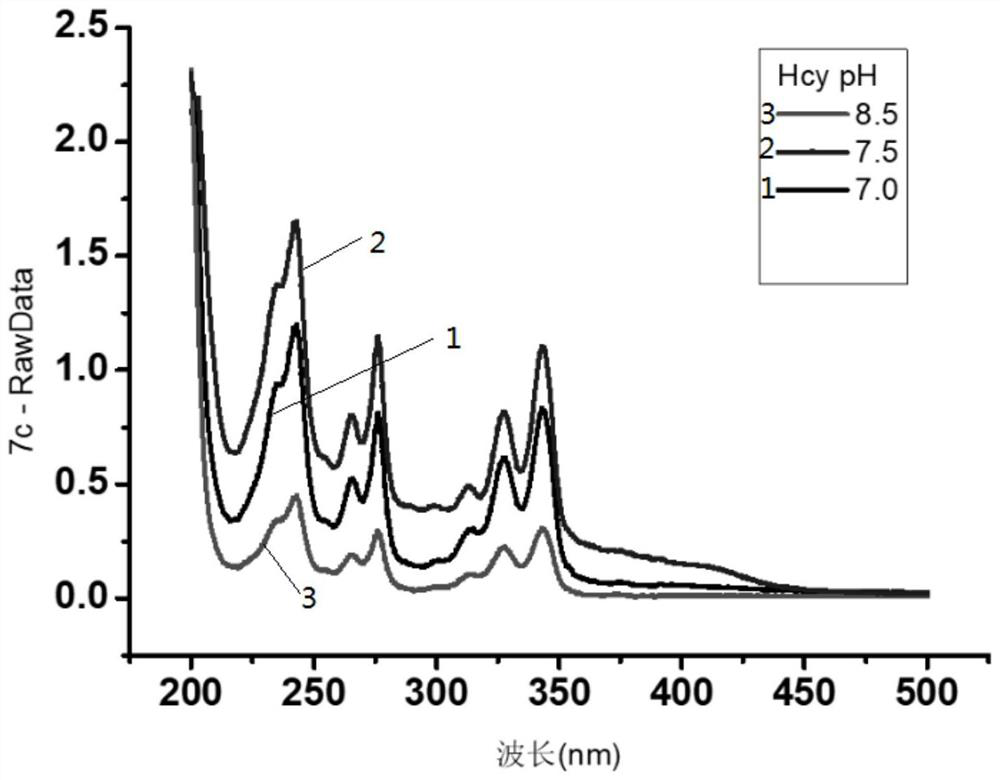
[0047] This embodiment provides 1-pyrene formaldehyde as the optimal condition test method for label derivatization of small biomolecules Cys and Hcy, which includes the following steps:
[0048] S1. Use PBS buffer solution to prepare Cys and Hcy stock solutions with a concentration of 5mM respectively, and store them in a refrigerator at 4°C for later use;
[0049] S2. Dissolving 1-pyreneformaldehyde in methanol to prepare a 1-pyreneformaldehyde solution with a concentration of 5mM.
[0050] S3. Take 500 μL of 1-pyrene formaldehyde solution in a 1.5 mL centrifuge tube, then add 250 μL of Cys and Hcy stock solutions obtained in step S1, and react to obtain a reaction solution.
[0051] S4, using an ultraviolet spectrophotometer to measure the absorbance of the reaction solution.
[0052] As a comparison: the pH of the PBS buffer solution in step S1 is 7, 7.5 and 8 respectively, and the test results are as follows Figure 1~2 As shown, it can be seen from the figure that the ...
Embodiment 2
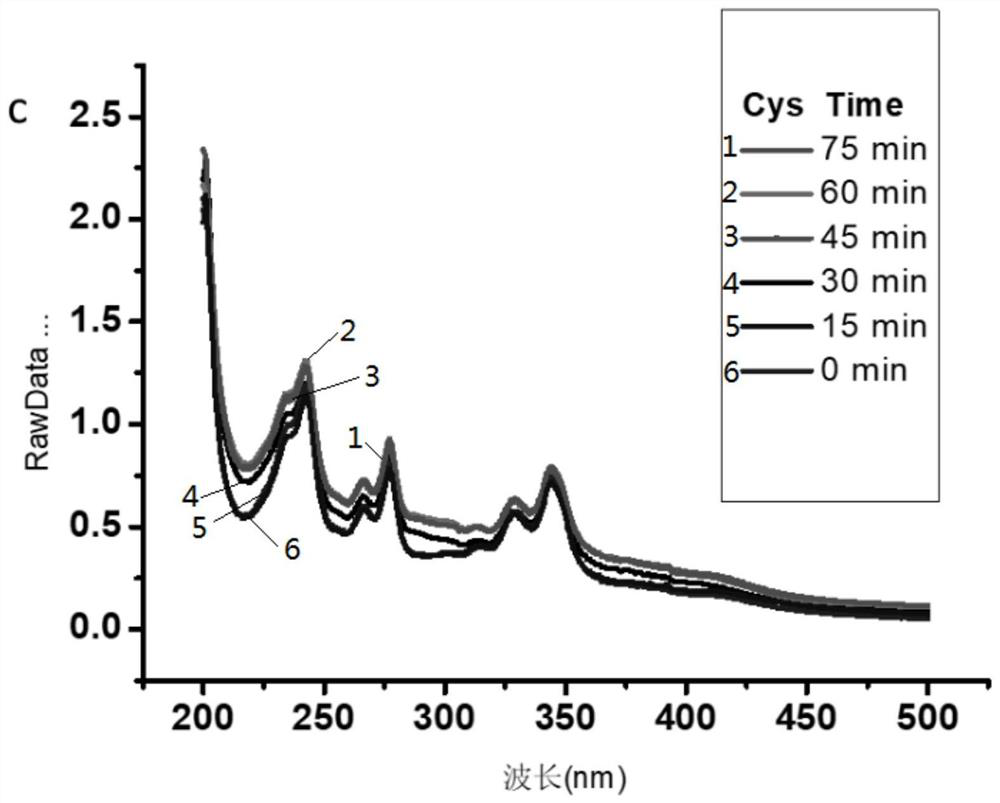
[0056] This embodiment provides the method that 1-pyrene formaldehyde is used as label detection biological small molecule Cys and Hcy in MALDI mass spectrometry, and it comprises the following steps:
[0057] S1. Cys and Hcy stock solutions with a concentration of 5 mM were prepared respectively using PBS buffer solution with pH=7.5, and stored in a refrigerator at 4° C. for future use.
[0058] S2. Dissolving 1-pyreneformaldehyde in methanol to prepare a 1-pyreneformaldehyde solution with a concentration of 5mM.
[0059] S3. Take 500 μL of 1-pyrene formaldehyde solution in a 1.5 mL centrifuge tube, then add 250 μL of the stock solution obtained in step S1, and react at room temperature for 1 hour to obtain a reaction solution.
[0060] S4, the substrate 2,5-dihydroxybenzaldehyde (DHB) is dissolved in the mixed solution of ammonium dihydrogen phosphate (ADP) and acetonitrile (ACN) with a volume ratio of 7:3, and the substrate solution whose concentration of DHB is 10g / L is pr...
Embodiment 3
[0066] This embodiment provides a method for measuring the detection limit of Cys and Hcy when 1-pyrene formaldehyde is used as a label, which includes the following steps:
[0067] S1. Dilute the stock solutions of Cys and Hcy in Example 2 to concentrations of 1 mM, 0.5 mM, 0.05 mM, 0.005 mM and 0.000000005 mM respectively according to the concentration gradient, and set aside.
[0068] S2. Dissolving 1-pyreneformaldehyde in methanol to prepare a 1-pyreneformaldehyde solution with a concentration of 5mM.
[0069] S3. Take five 1.5mL centrifuge tubes numbered 1, 2, 3, 4, and 5, add 500 μL of 1-pyrene formaldehyde solution, and then add 500 μL of Cys and Hcy solutions of different concentrations in step S1, and react at room temperature for 1 hour , to obtain the reaction solution.
[0070] S4, the substrate 2,5-dihydroxybenzaldehyde (DHB) is dissolved in the mixed solution of ammonium dihydrogen phosphate (ADP) and acetonitrile (ACN) with a volume ratio of 7:3, and the substr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com