Drug-loaded micelles with particle size reduction and surface charge reversal in response to acidic microenvironment and preparation method thereof
A technology of drug-loaded micelles and particle size, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as limited tumor penetration ability, and improve anti-tumor effects. , Improve the ability of penetration, the effect of long cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
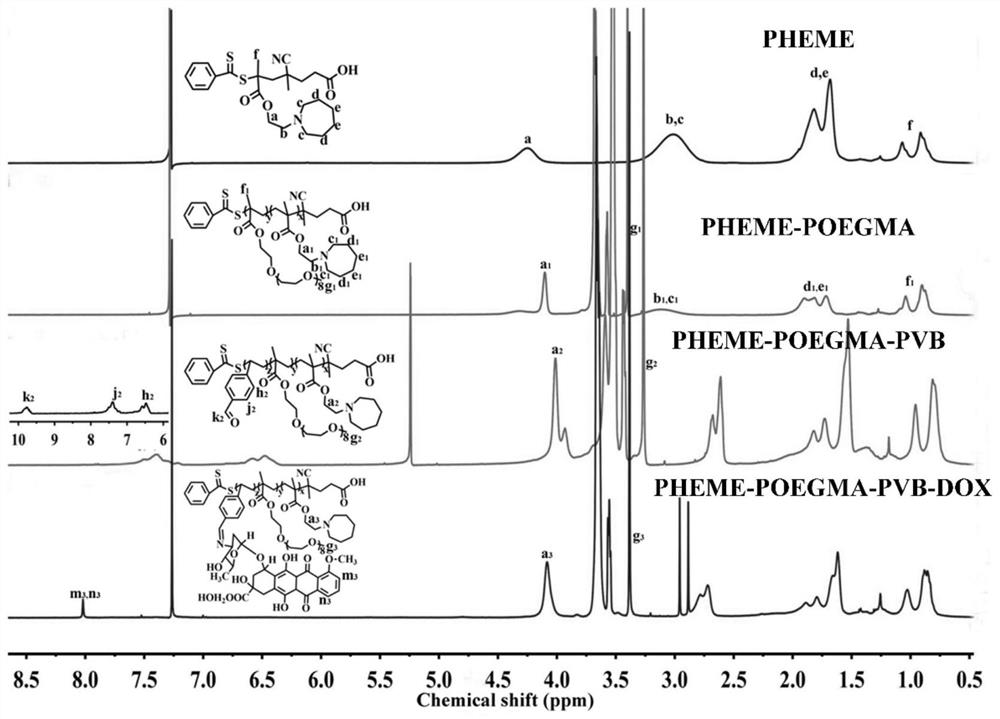
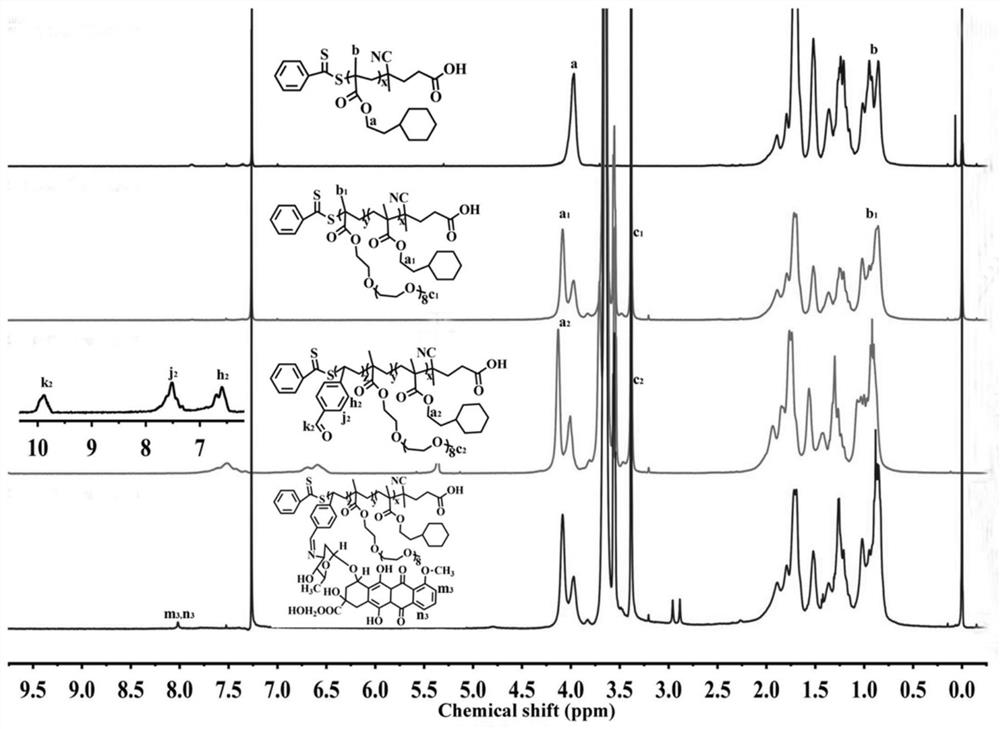
[0052] In this embodiment, the polymer prodrug is synthesized, and the synthetic route and steps are as follows:
[0053]
[0054] (1) Chain transfer agent 4-cyano-4-(thiobenzoyl)valeric acid (CPDB), 2-(hexamethyleneimine) ethanol methacrylate (HEME) monomer and initiator azo Diisobutyronitrile (AIBN) was dissolved in redistilled 1,4-dioxane (1,4-Dioxane), the molar ratio of HEME to CPDB was 80:1, and the molar ratio of AIBN to CPDB was 0.2: 1. The concentration of CPDB in 1,4-dioxane is 0.02mmol / mL. The resulting mixed solution was transferred to a pre-deoxygenated reaction bottle, followed by freezing-vacuum-nitrogen circulation three times to remove the oxygen in the reaction bottle, and then nitrogen protection and sealing the reaction bottle, and polymerized at 65 °C reaction, after 24 hours of polymerization reaction, the reaction was quenched with liquid nitrogen, dichloromethane was added to the obtained reaction liquid to dilute and precipitate in excess cold hexa...
Embodiment 2
[0075] In this example, the drug-loaded micelles were prepared on the basis of the polymer prodrug synthesized in Example 1, and the steps were as follows:
[0076] Take 3 mg of the polymer prodrug prepared in Example 1 and dissolve it in 200 μL of DMF to form a polymer prodrug solution, and slowly drop the polymer prodrug solution into 3 mL of pH=7.4 under ice bath and ultrasonic conditions with a power of 200 W. After the dropwise addition, continue to sonicate for 5 minutes in an ice bath, and then transfer to PBS with pH=7.4 to fully dialyze to remove DMF to obtain drug-loaded micelles.
[0077] Take 3 mg of the polymer prodrug prepared in Example 1 and dissolve it in 200 μL of DMF to form a polymer prodrug solution, and slowly drop the polymer prodrug solution into 3 mL of pH=6.7 under ice bath and ultrasonic conditions with a power of 200 W. After the dropwise addition, continue to sonicate for 5 minutes in an ice bath, and then transfer to PBS with pH=6.7 to fully dialy...
Embodiment 3
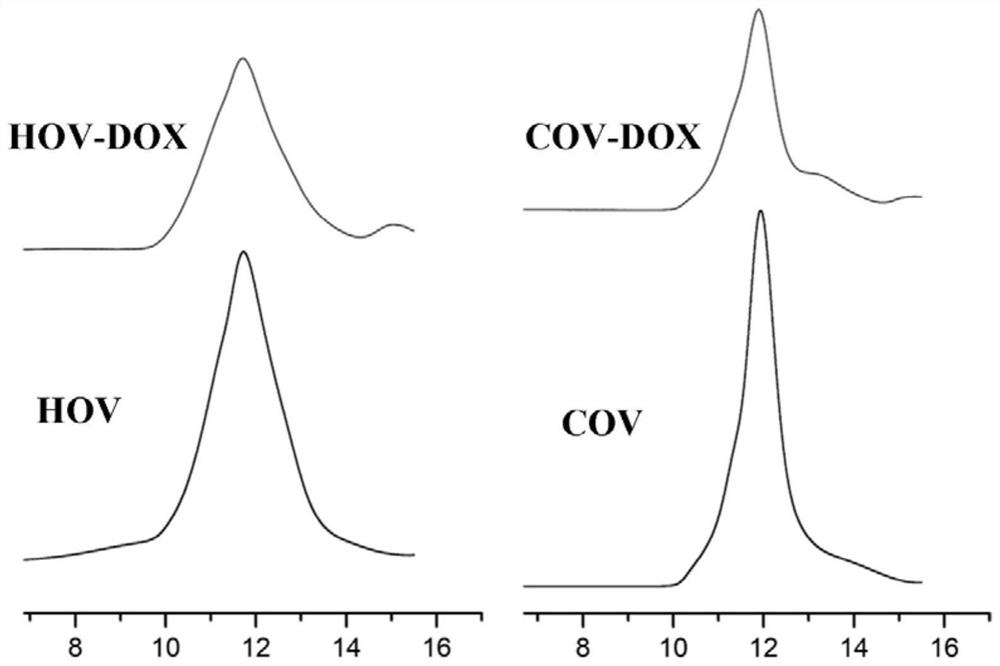
[0083] In this example, the particle size of the drug-loaded micelles prepared in Example 2 and Comparative Examples 3-4 was characterized by dynamic light scattering (DLS). Take the drug-loaded micelles prepared in Example 2 and Comparative Examples 3-4 under different pH conditions, and use PBS with corresponding pH values to prepare drug-loaded micellar solutions with a concentration of 1 mg / mL and pH values of 7.4 and 6.7, respectively. , the particle size of drug-loaded micelles was characterized by dynamic light scattering test. Measuring conditions: temperature 25°C, scattering angle 173°, each sample was measured three times. The results are shown in Table 2.
[0084] The drug-loaded micelles prepared in Example 2 and Comparative Example 3 were diluted with PBS of corresponding pH value to a drug-loaded micelle solution with a concentration of 100 μg / mL, and the drug-loaded micelles were characterized by transmission electron microscope (TEM) and atomic force micr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com