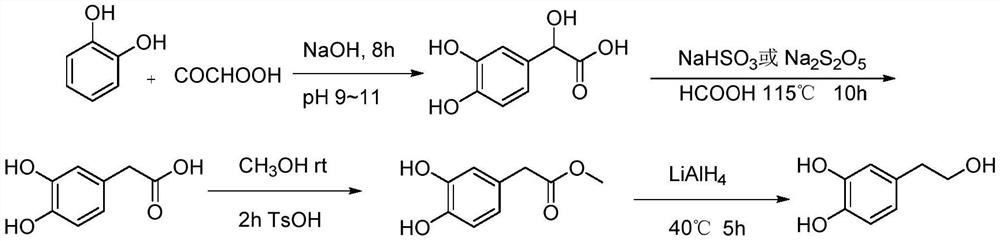
Synthesis method for hydroxytyrosol
A synthesis method and technology for hydroxytyrosol, applied in chemical instruments and methods, preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc. It can solve the problems of solvent residue, good selectivity and easy post-treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
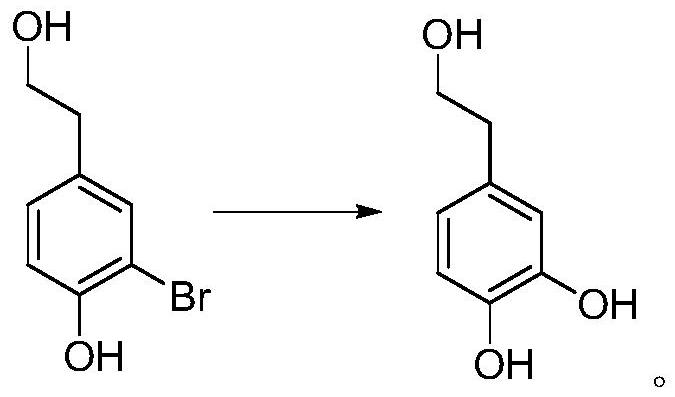
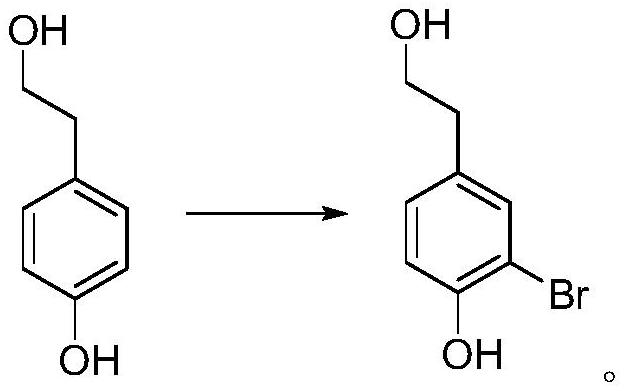
[0053] 1, the preparation of 3-bromo-4-hydroxyphenethyl alcohol
[0054]
[0055] Add 69.1g (0.5mol) of 4-hydroxyphenethyl alcohol, 500mL of water, and 200mL of acetone into the three-neck flask respectively. Install an ice-water bath, lower the temperature to below 15°C, and add 250 g (0.407 mol) of potassium monopersulfate compound salt in batches. Slowly add a pre-prepared 50 wt% sodium bromide aqueous solution (containing 51.5 g of sodium bromide, 0.5 mol) dropwise, and control the temperature below 20°C. After the dropwise addition was completed, the reaction was continued for 1 hour in an ice-water bath. TLC analysis (developing agent PE: EA = 2: 1), after the reaction is completed, slowly add 39.5 g (0.25 mol) of sodium thiosulfate in batches under an ice-water bath to quench the reaction, after the addition is complete, pour the reaction solution into In the single-necked bottle, acetone was evaporated under reduced pressure below 45°C, crystals were precipitated,...
Embodiment 2
[0064] 1, the preparation of 3-bromo-4-hydroxyphenethyl alcohol
[0065] Add 1mol 4-hydroxyphenylethanol, 1000mL water, and 400mL acetone into the three-neck flask respectively. Install an ice-water bath, lower the temperature to below 5°C, and add 2 mol of potassium monopersulfate compound salt in batches. Slowly add a pre-prepared 50 wt% potassium bromide aqueous solution (containing 2 mol of potassium bromide) dropwise, and control the temperature below 5°C. After the dropwise addition was completed, the reaction was continued for 2 hours in an ice-water bath. TLC analysis (developing agent PE: EA = 2: 1), after the reaction is completed, slowly add 0.8 mol of sodium thiosulfate in batches to quench the reaction under an ice-water bath, after the addition is completed, pour the reaction solution into a one-mouth bottle, The acetone was distilled off under reduced pressure below 45°C, and crystals were precipitated, filtered with suction, washed with water, and dried in a ...
Embodiment 3
[0069] 1, the preparation of 3-bromo-4-hydroxyphenethyl alcohol
[0070] Add 1mol 4-hydroxyphenylethanol, 1000mL water, and 500mL acetone into the three-neck flask respectively. Install an ice-water bath, lower the temperature to below 10°C, and add 1 mol of potassium monopersulfate compound salt in batches. Slowly add a pre-prepared 50 wt% sodium bromide aqueous solution (containing 3 mol of sodium bromide) dropwise, and control the temperature below 10°C. After the dropwise addition was completed, the reaction was continued for 3 hours in an ice-water bath. TLC analysis (developing agent PE: EA = 2: 1), after the reaction is completed, slowly add 1 mol of sodium bisulfite in batches to quench the reaction under an ice-water bath. Acetone was distilled off under reduced pressure below 45°C, and crystals were precipitated. Suction filtration, washing with water, and drying in a blast drying oven gave 0.8891 mol of the product, with a molar yield of 88.91%.
[0071] 2, Prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com