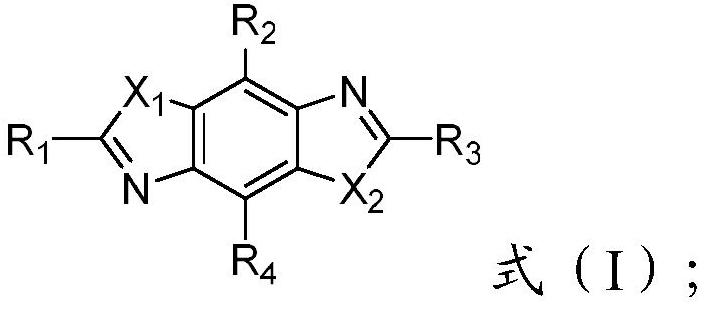
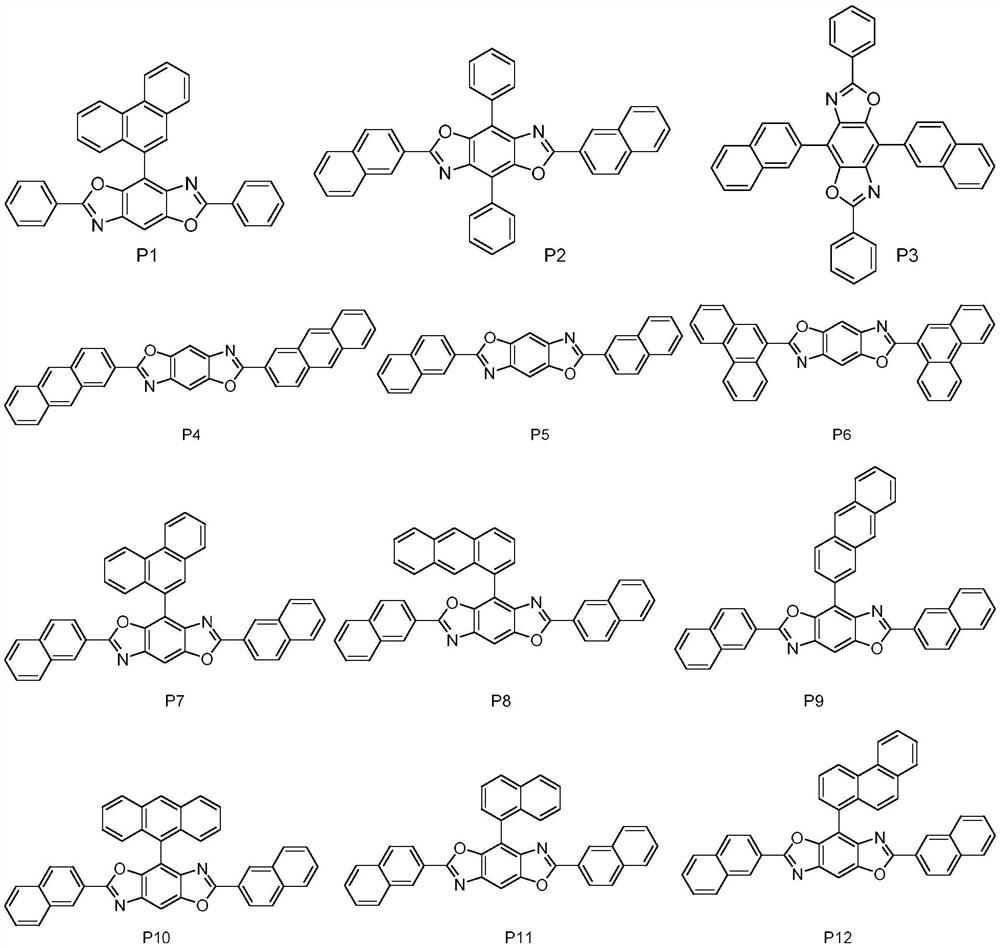
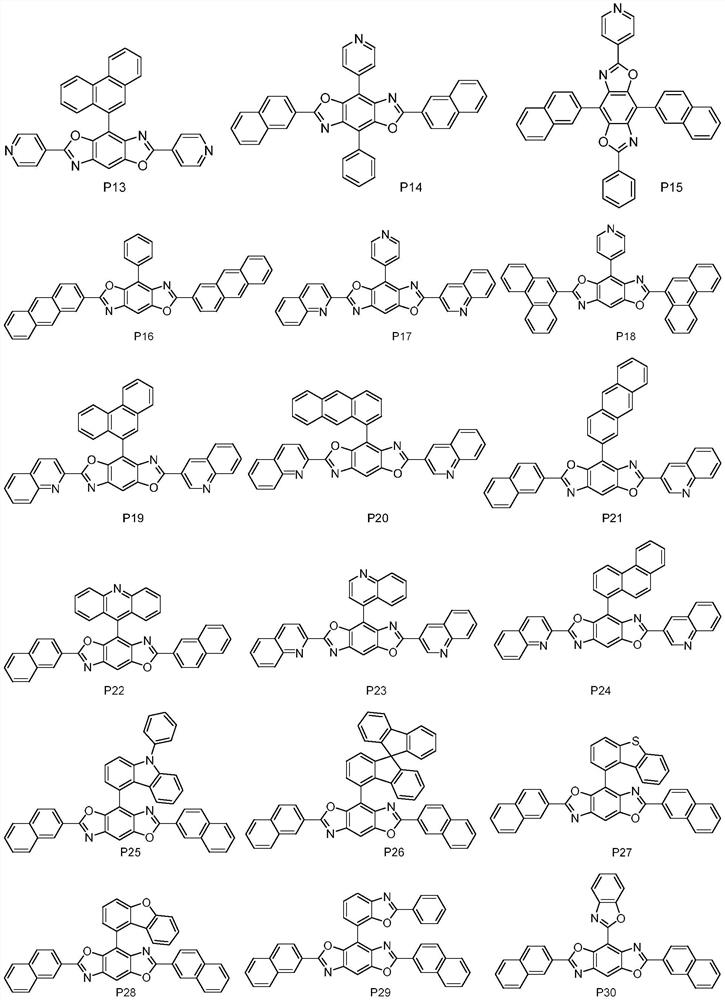
High refractive index benzoheterocycle compound, organic light-emitting device and display device
A high refractive index, compound technology, applied in the field of organic electroluminescent materials, can solve problems such as poor coverage tightness, unfavorable light extraction, weak luminous efficiency, etc., to improve external quantum efficiency, alleviate angle dependence, and improve luminous efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The synthetic route is as follows:
[0071]
[0072] Concrete preparation method specifically comprises the following steps:
[0073] (1) P2-1 (3.0 mmol), 2-naphthoic acid (7.0 mmol), and polyphosphate (16 g) were mixed, put into a 100 mL flask, and reacted at 150° C. for 24 hours. Cool to room temperature, then slowly add 1M sodium hydroxide solution to the solution for neutralization, then filter out the solid particles, wash the dark solid with water, ethanol, and ethyl acetate, and then air-dry to obtain the crude product P2-2.
[0074] Test the structure of the target product P2-2: MALDI-TOF MS (m / z) obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis: C 28 h 14 Br 2 N 2 o 2 , the calculated value is 567.9, and the tested value is 568.0.
[0075] (2) In a 100mL round bottom flask, the intermediate product P2-2 (15mmol) and potassium acetate (30mmol) were mixed with dry 1,4-dioxane (60mL), Pd(PPh 3 ) 2 Cl 2 (...
Embodiment 2
[0081] The synthetic route is as follows:
[0082]
[0083] Concrete preparation method specifically comprises the following steps:
[0084] (1) P20-1 (3.0mmol), P20-2 (7.0mmol) and polyphosphate (16g) were mixed, put into a 100mL flask, and it reacted at 150 degreeC for 24 hours. Cool to room temperature, then slowly add 1M sodium hydroxide solution to the solution for neutralization, filter out the solid particles, wash the dark solid with water, ethanol, and ethyl acetate, and then air-dry to obtain the crude product P20-3.
[0085] Test the structure of the target product P20-3: MALDI-TOF MS (m / z) obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis: C 26 h 13 BrN 4 o 2 , the calculated value is 492.0, and the tested value is 492.1.
[0086] (2) In a 100mL round bottom flask, the intermediate product P20-3 (15mmol) and potassium acetate (20mmol) were mixed with dry 1,4-dioxane (60mL), Pd(PPh 3 ) 2 Cl 2 (0.50mmol) and ...
Embodiment 3
[0092] The synthetic route is as follows:
[0093]
[0094] (1) In a 100mL round bottom flask, P24-1 (10mmol), P24-2 (12mmol) and Pd(PPh 3 ) 4 (0.5mmol) was added to a mixture of toluene (30mL) / ethanol (20mL) and potassium carbonate (25mmol) aqueous solution (10mL), and the reaction was refluxed under nitrogen atmosphere for 12h. The resulting mixture was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude The product yielded the final product P24.
[0095] Test the structure of the target product P24: MALDI-TOF MS (m / z) obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis: C 40 h 22 N 4 o 2 , the calculated value is 590.2, and the tested value is 590.1.
[0096] Elemental analysis: theoretical value C, 81.34; H, 3.75; N, 9.49; test value C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com