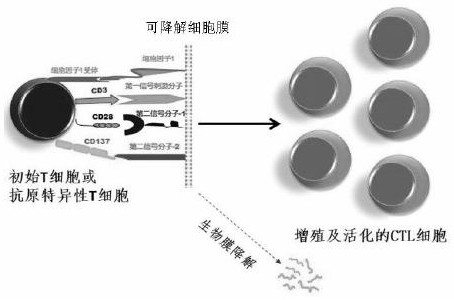
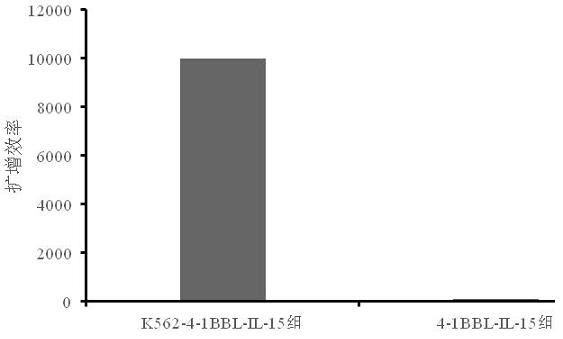
Cell membrane for rapid amplification of NK cells and application thereof
A NK cell and cell membrane technology, applied in the field of biomedicine, can solve the problem of low NK cell expansion efficiency, reduce the loss and pollution probability, promote in vitro proliferation, and improve the effect of expansion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis and construction of IL-15 and 4-1BBL expression plasmids:
[0040] The nucleic acid and amino acid sequences of IL-15 and 4-1BBL were obtained from the NCBI database, and IL-15 and 4-1BBL molecules were synthesized by the method of total gene synthesis, and then the obtained IL-15 sequences and 4-1BBL molecules were respectively Constructed into pDNA3.1 molecule by double enzyme digestion method, the transfection-positive strains were expanded and cultured, and the pDNA3.1-IL-15 and 4-1BBL plasmids were extracted using the endotoxin-free plasmid extraction kit.
[0041] Nucleic acid sequence: (IL-15+ linker +transmembrane protein)
[0042]gaattcgccgccaccATGGAGTTCGGACTCAGTTGGCTGTTCCTGGTGGCCATCCTGAAGGGTGTGCAGTGATGAGAATTTCGAAACCACATTTGAGAAGTATTTCCATCCAGTGCTACTTGTGTTTACTTCTAAACAGTCATTTTCTAACTGAAGCTGGCATTCATGTCTTCATTTTGGGCTGTTTCAGTGCAGGGCTTCCTAAAACAGAAGCCAACTGGGTGAATGTAATAAGTGATTTGAAAAAAATTGAAGATCTTATTCAATCTATGCATATTGATGCTACTTTATATACGGAAAGTGATGTTCACCCCAGTTGCAAAGT...
Embodiment 2
[0051] Cultured transfection of K562 cells:
[0052] Recovery and culture of K562 cells:
[0053] The original cell of K562 is a malignant tumor cell of the hematopoietic system with multilineage differentiation potential, which can spontaneously differentiate into identifiable progenitor cells of erythroid, myeloid and monocytic. Culture conditions: 1640 medium 10% FBS, subculture method: maintain cell density at 10 5 -10 6 Each / ml, change the medium 2-3 times a week.
[0054] Electroporation:
[0055] Use BIO-RAD's electroporator to electroporate the plasmids of pDNA3.1-IL-15 and 4-1BBL, the steps are as follows:
[0056] For a 0.2 cm electroporation cup, the cell density is 10x10^6 cells / ml, the amount of DNA is 2 μg, the volume of electrotransfer solution is 100 μl, the voltage is 130 V, and the capacitance is 950 μF. After electroporation, 1640 medium was quickly added, and the cells were transferred to culture flasks to resume culture.
Embodiment 3
[0058] Preparation of cell membranes for NK cell expansion
[0059] Grow at K562 cell density up to 10 9 -10 10 cells / ml, collect the cells, wash the cells with PBS, add 10mM tris hydrochloric acid solution, stir at 4°C for 2 hours to break the cells, then centrifuge at low temperature for 30 minutes, collect the precipitate, wash 3 times with 10mM tris hydrochloric acid solution, After centrifugation at low temperature and high speed for 10 minutes, the pellet was resuspended with PBS to obtain the material of K562 cell membrane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| PCR efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com