A kind of preparation method of lithium sulfide, lithium sulfide and application thereof
A technology of lithium sulfide and anhydrous sodium sulfide, applied in the direction of alkali metal sulfide/polysulfide, lithium battery, structural parts, etc., can solve the problem of high cost of lithium sulfide preparation, and achieve suitable mass production and environmental protection Friendly, reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
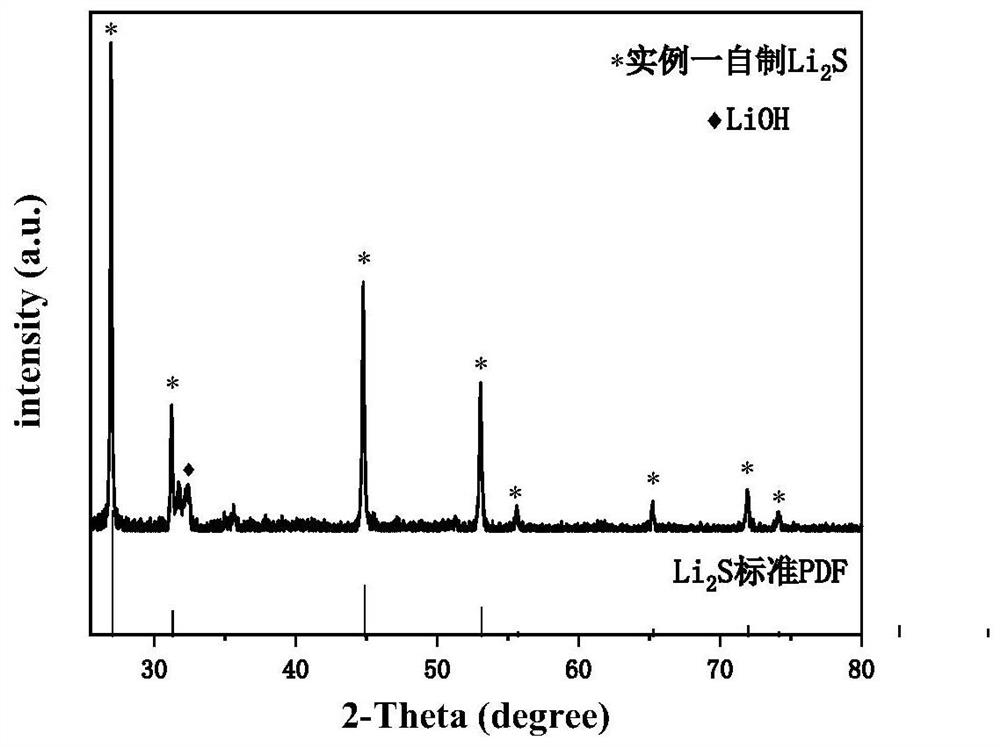
example 1
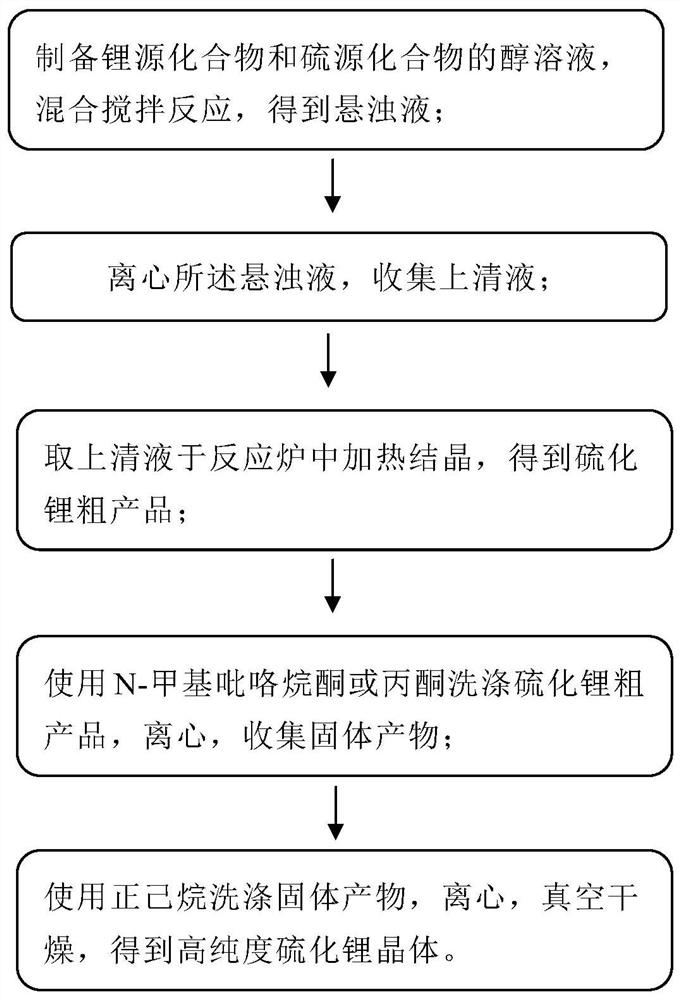
[0036] Method for preparing sulfide, including the following steps:
[0037] S1, under argon atmosphere protection, sodium dehydrated anhydrous sodium sulfide (NA) 2 S) and lithium chloride (LiCl) raw materials Mass mole ratio 0.5: 2. In the argon atmosphere glove box, sodium sulfide mass was 102 mg, 10 ml of anhydrous ethanol was added. The container is placed on a magnetic stirrer and the rotational speed is 520 rpm, and the stirring time is 30 min. The lithium chloride mass was 185 mg, and 4 ml of absolute ethanol magnet was stirred, the rotational speed was 520 rpm, and the stirring time was 20 min. The configured lithium chloride / ethanol solution was added to the sodium sulfide / ethanol solution, and stirred while stirring, a white precipitate was stirred, continuously stirred for 12 h, and the rotational speed was 520 rpm to obtain a suspension. Among them, the reactive reaction between sodium sulfide and lithium chloride is:
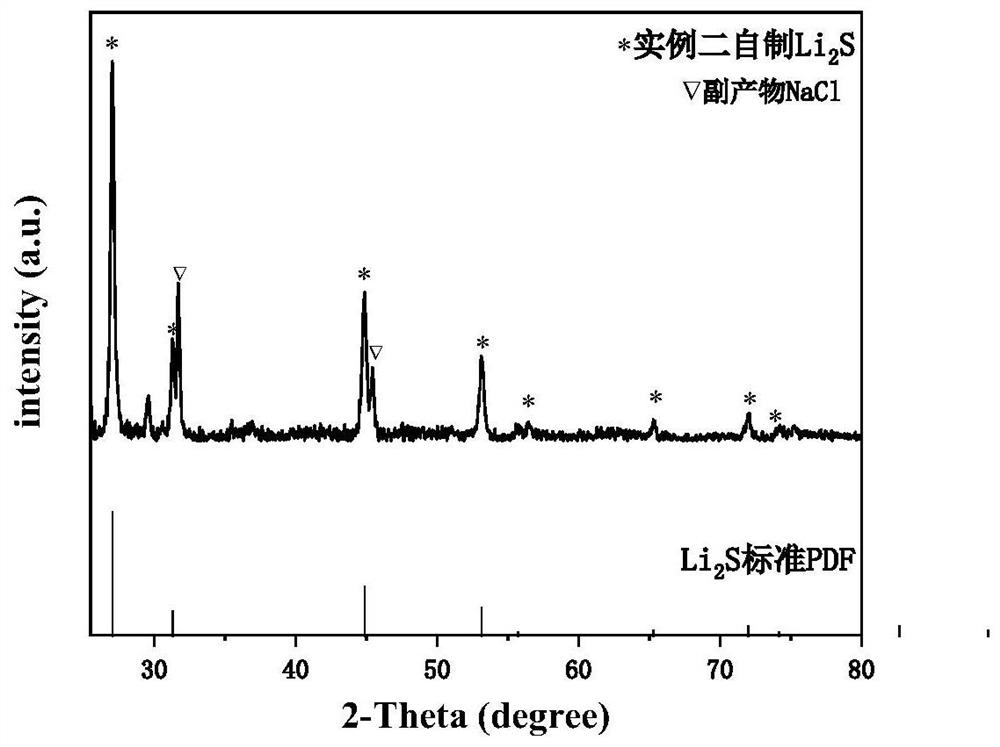
example 2
[0044] Method for preparing sulfide, including the following steps:
[0045] S1, 5g nine water sodium sulfide (NA 2 S · 9h 2 O) The raw material is heated in the muffle furnace of nitrogen protection, and the temperature is set to 300 to 350 ° C, and the time is set to 6 to 12 hours to give 1.6 g of anhydrous sodium sulfide. Under a nitrogen atmosphere, anhydrous sodium sulfide and lithium chloride raw materials were measured from 1: 2. In the nitrogen atmosphere glove box, the sodium sulfide mass was measured at 170 mg, 10 ml of anhydrous ethanol was added. The container is placed on a magnetic stirrer and the rotation speed is 720 rpm, and the stirring time is 60 min. The lithium chloride mass was 185 mg, and 4 ml of absolute anhydrous ethanol was stirred, the rotational speed was 720 rpm, and the stirring time was 15 min. The configured lithium chloride / ethanol solution was added to a sodium sulfide / ethanol solution, and stirred while stirring, a white precipitate was forme...
example 3
[0052] Method for preparing sulfide, including the following steps:
[0053] S1, under argon atmosphere protection, dried dehydrated anhydrous sodium sulfide and lithium chloride raw materials were measured from 0.8: 2. In the argon atmosphere glove box, the sodium sulfide mass was 136 mg, 8 ml of anhydrous ethanol was added. The container is placed on a magnetic stirrer and the rotation speed is 900 rpm, and the stirring time is 30 min. It is weighed with a lithium chloride mass of 190 mg, and 4 ml of anhydrous ethanol magnetic stirring was added, the rotational speed was 900 rpm, and the stirring time was 20 min. The configured lithium chloride / ethanol solution was added to the sodium sulfide / ethanol solution, and stirred while stirring, it immediately had a white precipitate, continuously stirred for 12 h, and the rotation speed was 500 rpm, and the suspension was obtained. Among them, the reactive reaction between sodium sulfide and lithium chloride is:
[0054] NA 2 S (SO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com