Polypeptide and application thereof
An amino acid and N-terminal technology, applied in the field of biomedicine, can solve problems such as short half-life, increased medical expenses, and inability to completely reverse islets, and achieve high stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
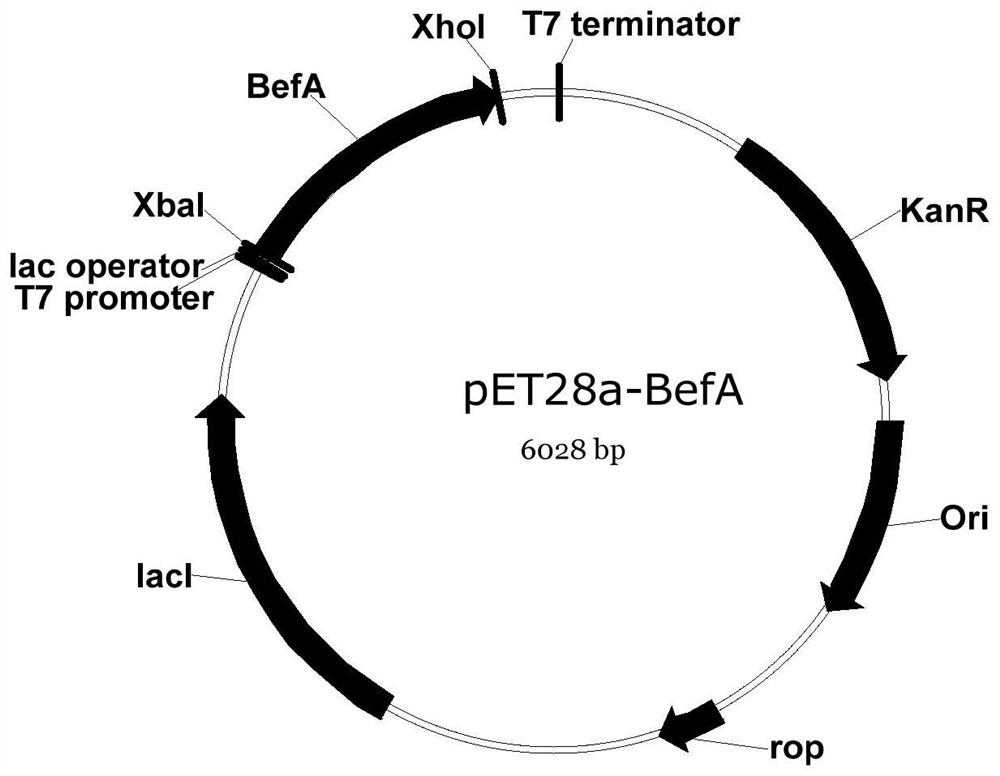
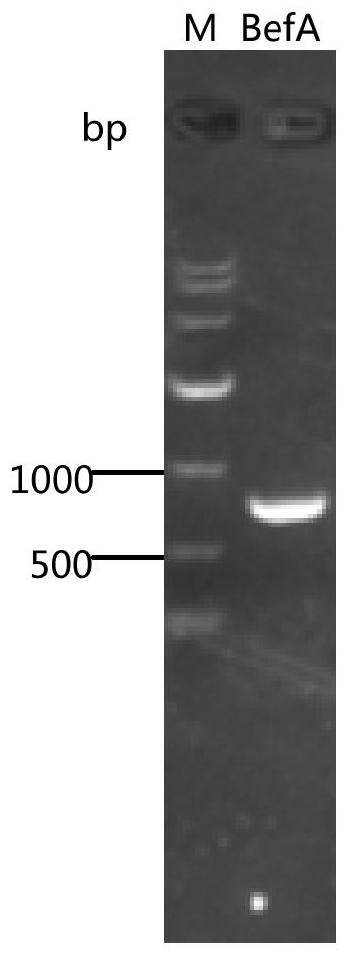
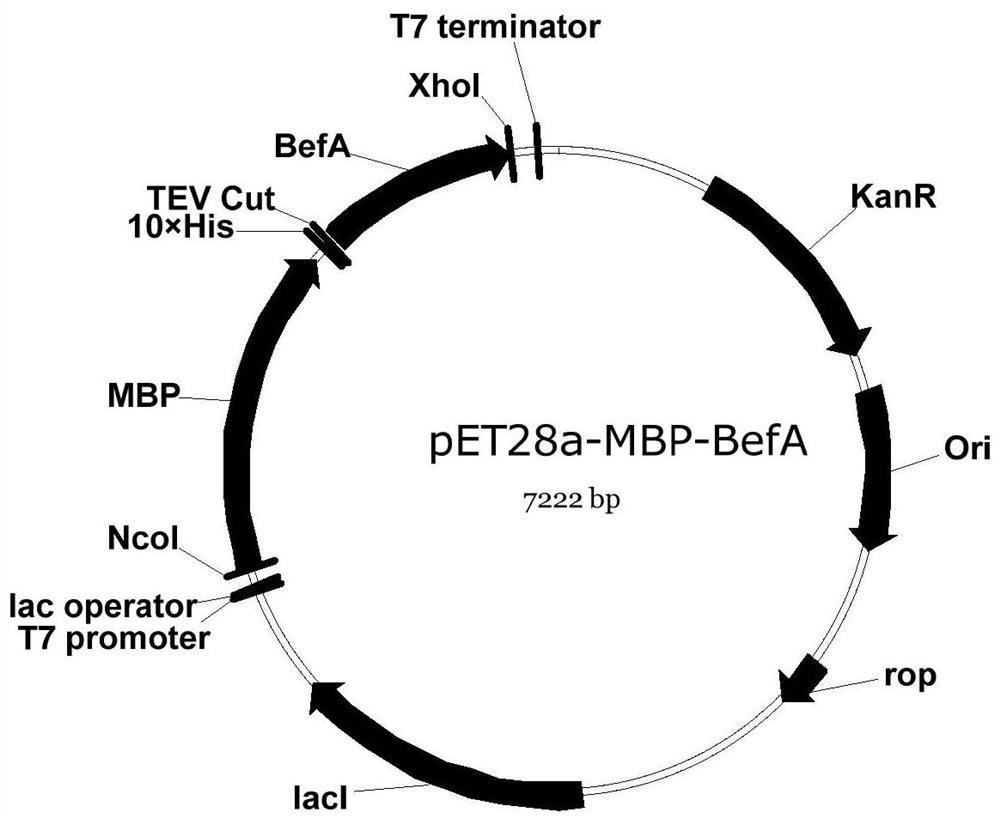
[0084] Example 1 Recombinant expression plasmid PET28A-BEFA, PET28A-MBP-B construction
[0085] 1, β cell proliferative factor A (BEFA), all gene synthesis of MBP partner protein
[0086] The person literature (Jennifer Hampton Hill, A conserved bacterial protein inducespancreatic beta cell expansion during zebrafish development, elifesciences, Dec.2016) gut BefA published amino acid sequence (co 258aa) secreted by strain Enterococcus gallinaru, according to E. coli (BL21 ( The optimization principle of DE3)) is optimized for nucleic acid sequences. At the same time, the sequence is strictly defined, and the sequence 3 'end adds a termination codon (TAA) to obtain the encoding gene BEFA, and finally handed over to the gene synthesis company for all gene synthesis (PUC57-BEFA).
[0087] The MBP partner gene is encoded containing a TEV (cysteine protease) enzyme disaster in TEV (tobacco corrusor virus), and the nucleic acid sequence is optimized according to the optimization princ...
Embodiment 2
[0092] Example 2 Engineering Cell Expression Production BEFA / Befa Mutant
[0093] 1, shake flask fermented befa / mbp-befa protein
[0094] The positive strain glycerol tube obtained by screening and identifying 0.2% inoculated to 5 ml of 50 μg / ml kanamycin LB liquid medium, 37 ° C 250 rpm activated overnight; inoculated by 2% inoculation to 50 μg / ml In the 2000ml triangular bottle of kanamycin LB liquid medium, the amount of liquid is 20%, 37 ° C 250 rpm to OD 600 When = 0.6 ~ 0.8, IPTG was added to 1 mm, and the final concentration was 1 mM, and the expression of 4 to 6 h was induced at 37 ° C; the fermentation broth was centrifuged (8000 rpm 10 min), and the bacteria was collected. After the purified water resuspended, the bracket (ultrasonic crushing or homogenization is broken), and the fragmentation is purified by low temperature high speed (12000 rpm 30min 4 ° C), and the supernatant is obtained.
[0095] 2, purify the Befa protein
[0096] BEFA protein uses nickel pi...
Embodiment 3
[0120] Example 3 BEFA and BEFA mutant activity detection
[0121] 1, INS-1 cell proliferation experiment
[0122] INS-1 (rat inside islet cell tumor cell) cells Press 3 * 10 4 ~ 6 * 10 4 Cells / ML, Press 100 μL / Well to 96-well plate, 37 ° C, 5% CO 2 Culture 48h. Exchange to sugar-free 1640 + 0.1% BSA, hunger 24h. Discard the hunger medium, add 1640 + 1% FBS medium dilution to 10 -9 M ~ 10 -7 M'S BEFA Protein (or Befa Mutant Befa Modification) Stimulation, 100μL / Well. 37 ° C, 5% CO 2 Culture 72h. Add CCK8 reagent, 10 μL / Well, 37 ° C, 5% CO 2 After 2 hours of incubator, detection OD 450 . See Figure 7-8 . BEFA proteins and BEFA mutants can significantly promote proliferation of INS-1 cells.
[0123] 2, mouse pharmacodynamic experiment
[0124] (1) BEFA protein OB / ob mouse pharmacodynamic experiment
[0125] Ob / OB mouse divided by 8 animals per group, 30 mg / kg of Befa protein and PBS buffer per group, 1 time per day, 4 weeks of administration, oral sugar at the 7th day ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com