Method for recycling fluorine-containing sulfuric acid
A technology of sulfuric acid and hydrofluoric acid, applied in the direction of sulfur trioxide/sulfuric acid, hydrogen fluoride, fluorine/hydrogen fluoride, etc., can solve the problems of waste of fluorine resources, high reaction temperature, low concentration of sulfuric acid, etc., and achieve low equipment corrosion, mild conditions, The effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Under normal pressure, add 15% deionized water into the reaction kettle equipped with fluorine-containing sulfuric acid, then pass in pure nitrogen and heat to 110°C, treat for 6.5h, during this process, pass the tail gas generated into In the deionized water tank, the temperature of the deionized water tank was kept at 10°C.
[0043] After the reaction was completed, leave fluorine-free sulfuric acid in the final reactor, and the concentration of sulfuric acid is 96%; the content of hydrofluoric acid is below 10ppm; Below 20ppm.
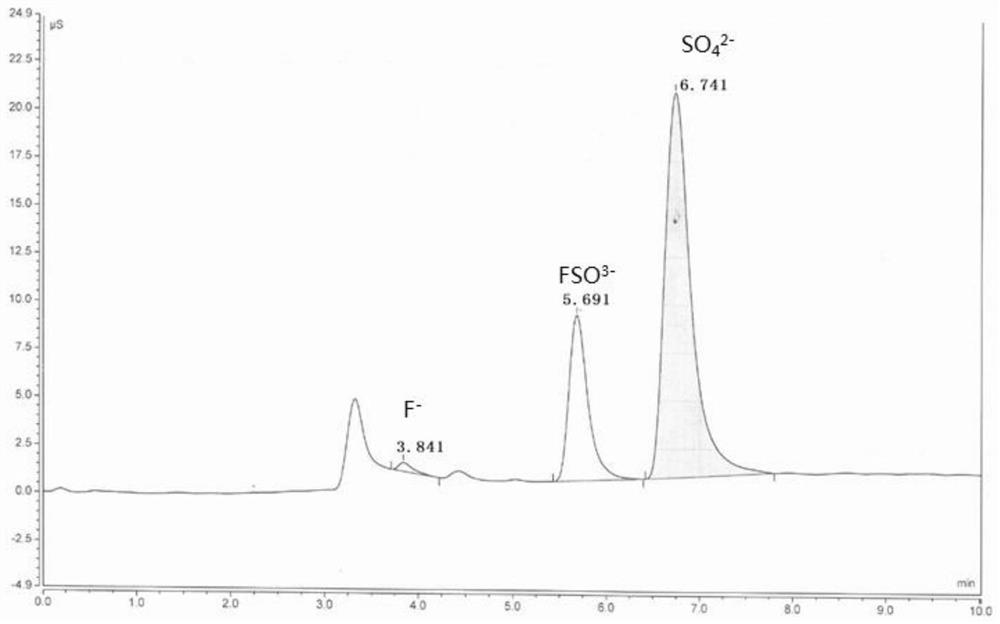
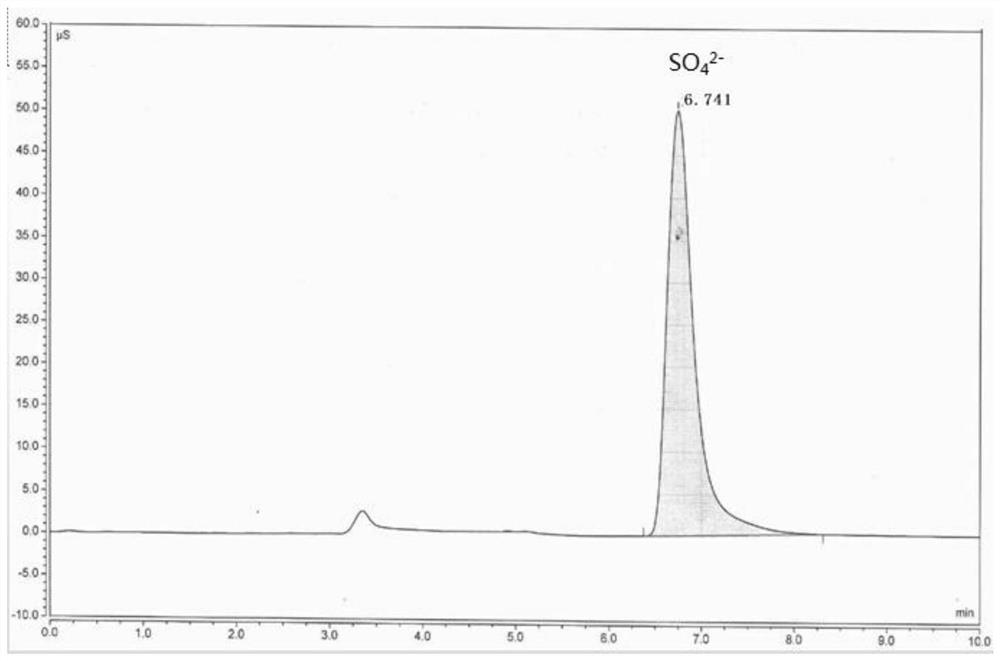
[0044] like figure 1 It is the ion chromatogram before fluorine-containing sulfuric acid is processed by the inventive method, figure 2It is an ion chromatogram of fluorine-containing sulfuric acid treated by the method of the present invention. As can be seen from the comparison of the two figures, after the fluorine-containing sulfuric acid is processed by the method of the present invention, figure 2 F peak and FSO have not been dete...
Embodiment 2
[0046] Under normal pressure, feed pure nitrogen into the reaction kettle equipped with fluorine-containing sulfuric acid and heat it to 110°C for 12 minutes. Keep at 10°C. Then take a sample from the reactor, after 100-fold dilution, measure the FSO 3 - content, and then calculate the FSO in the fluorine-containing sulfuric acid in the reactor 3 - content, calculate the theoretical water amount M required for the complete hydrolysis of fluorosulfonic acid in all the fluorine-containing sulfuric acid in the reactor, add 1.05M water into the reactor, stir, then feed nitrogen and heat to 110°C, treat for 5h, During this process, the generated tail gas was passed into the deionized water tank, and the temperature of the deionized water tank was kept at 10°C.
[0047] After the reaction is completed, leave fluorine-free sulfuric acid in the final reactor, and the sulfuric acid concentration is 97%; the hydrofluoric acid content is below 10ppm; the hydrofluoric acid with a hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com