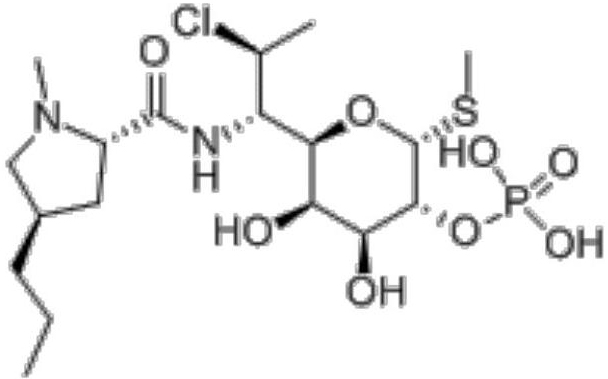
A kind of preparation method of clindamycin phosphate
A technology of clindamycin phosphate and clindamycin, which is applied in the field of preparation of clindamycin phosphate, can solve the problems of material transfer and dripping, hidden dangers of operation safety, and leakage of phosgene, etc., so as to improve the purity , The effect of small product damage and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
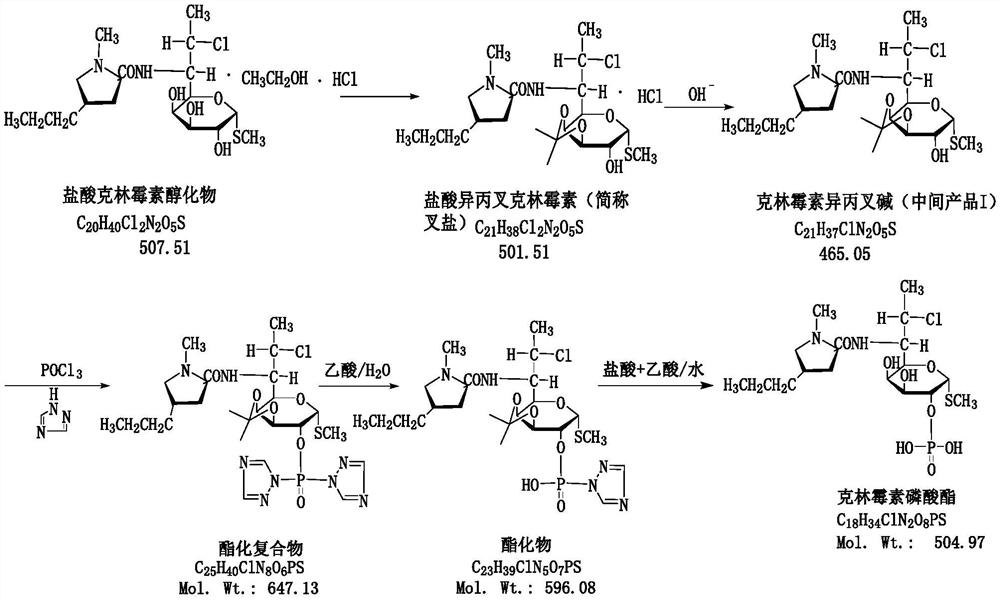
[0052] 1) Preparation of Clindamycin Isopropylidene
[0053] Add 120g (0.2364mol) of clindamycin hydrochloride alcoholate, 60g (0.405mol) of triethyl orthoformate to 480g of acetone, then add 1.8g (0.01mol) of p-toluenesulfonic acid, and keep warm at 40°C for 3 Hours; after the reaction is completed by pointing the board, cool down to 0°C, grow the crystal for 2 hours, filter, wash with acetone; add the obtained wet product to 200g purified water containing 26.5g sodium carbonate (0.25mol) and 200g acetone, and stir at 50°C After 30 minutes, the temperature was lowered to 20° C. to grow crystals for 2 hours, filtered, and then dried at 70° C. to obtain 105 g of clindamycin isopropylidene, with a yield of 95.0% and a purity of ≥99.2%.
[0054] 2) Preparation of Clindamycin Phosphate
[0055] Add 32.5g (0.47mol) of 1,2,4-triazole into 520g of dichloromethane, cool down to 0°C, then slowly add dropwise a dichloromethane solution containing 30.6g (0.2mol) of phosphorus oxychlorid...
Embodiment 2
[0059] 1) Preparation of Clindamycin Isopropylidene
[0060] Add 79g (0.15mol) of clindamycin hydrochloride alcoholate and 44g (0.3mol) of triethyl orthoformate to 290.4g of acetone, then add 0.86g (0.005mol) of p-toluenesulfonic acid, and keep the reaction at 45°C 3 hours; after the reaction is completed by pointing the plate, cool down to 0°C, grow the crystal for 2 hours, filter and wash with acetone; Stir for 30 minutes, then cool down to 20°C to grow crystals for 2 hours, filter, and then dry at 70°C to obtain 69 g of clindamycin isopropylidene, with a yield of 94.3% and a purity of ≥99.0%.
[0061] 2) Preparation of Clindamycin Phosphate
[0062] Add 27.6g (0.4mol) of 1,2,4-triazole into 420g of dichloromethane, cool down to 0°C, then slowly add dropwise a solution of dichloromethane containing 15.3g (0.1mol) of phosphorus oxychloride, dropwise After adding, slowly add the dichloromethane solution containing 46.5g (0.1mol) clindamycin isopropylidene base dropwise, then...
Embodiment 3
[0066] 1) Preparation of Clindamycin Isopropylidene
[0067] Add 152g (0.3mol) of clindamycin hydrochloride alcoholate and 74g (0.5mol) of triethyl orthoformate to 580g of acetone, then add 2.6g (0.015mol) of p-toluenesulfonic acid, and keep warm at 40°C for 3 Hours; after the reaction is completed by pointing the board, cool down to 0°C, grow crystals for 2 hours, filter, and wash with acetone; the obtained wet product is added to 253g of purified water containing 41.3g of sodium carbonate (0.39mol) and 253g of acetone, and stirred at 50°C After 30 minutes, the temperature was lowered to 20° C. to grow crystals for 2 hours, filtered, and then dried at 70° C. to obtain 132 g of clindamycin isopropylidene, with a yield of 95.0% and a purity of ≥99.2%.
[0068] 2) Preparation of Clindamycin Phosphate
[0069] Add 38g (0.55mol) of 1,2,4-triazole into 600g of dichloromethane, cool down to 0°C, then slowly add dropwise a solution of dichloromethane containing 38.3g (0.25mol) of ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com