Difluprednate suspension eye drops and preparation method thereof
A technology of difluprednate and eye drops, applied in the field of difluprednate suspension eye drops and its preparation, can solve the problem of increasing the workload of medical staff, unfavorable eye surgery wound healing, increased wound infection, etc. Problems, to achieve the effect of promoting wound healing, reducing drug concentration fluctuations, and prolonging residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
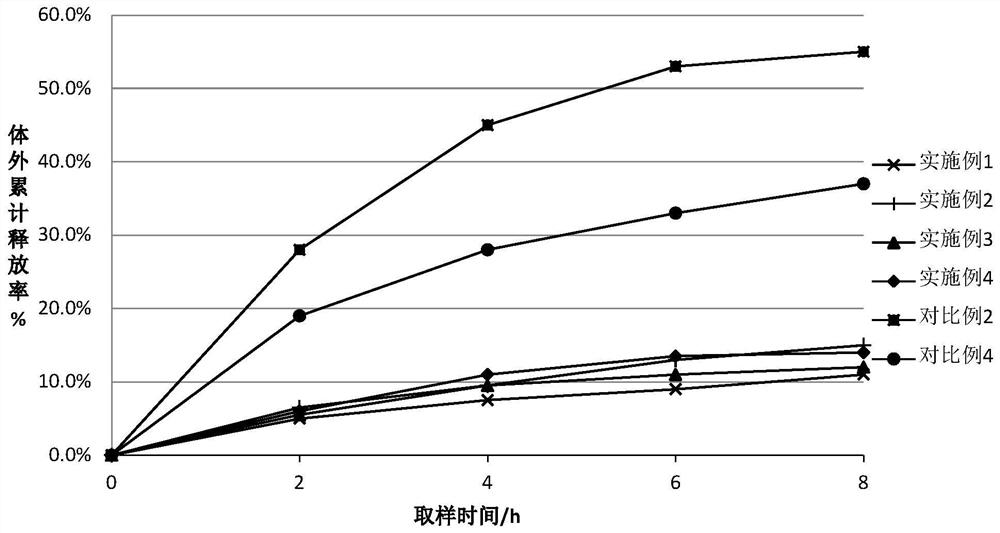
Embodiment 1~3、 comparative example 1~2
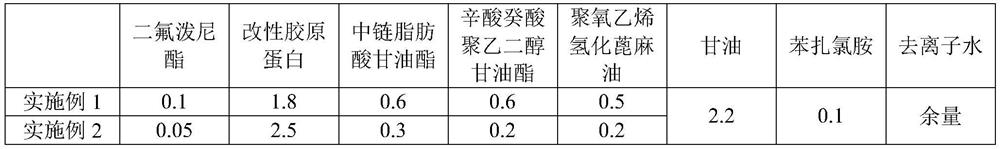
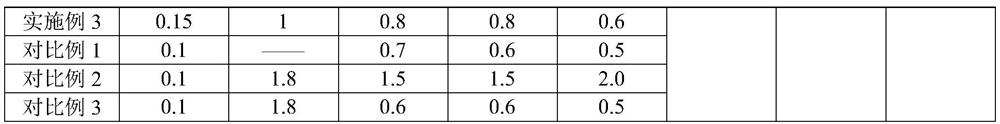
[0025] For the difluprednate eye drops provided in Examples 1-3 and Comparative Examples 1-2, the raw materials used are shown in Table 1 below by weight percentage.
[0026] The component list (%) of the difluprednate eye drops of table 1 embodiment 1~3 and comparative example 1~2
[0027]
[0028]
[0029] The difluprednate eye drops of above embodiment 1~3 and comparative example 1~2, its preparation method is:
[0030] S1, under nitrogen protection environment, difluprednate, medium-chain fatty acid glycerides, polyoxyethylene hydrogenated castor oil, caprylic capric acid macrogol glycerides are stirred at 50~55 ℃ for 20min to obtain the oil phase (with undissolved drug particles visible to the naked eye);
[0031] S2. Under a nitrogen protection environment, take 50% deionized water, benzalkonium chloride, and glycerin and stir at 55° C. for 8 minutes to obtain an aqueous phase;
[0032] S3. Under a nitrogen protection environment, slowly add the oil phase to the ...
Embodiment 4
[0043] The type and consumption of the raw materials of the difluprednate suspension eye drops provided in this embodiment are exactly the same as those in Example 1. The preparation method of the suspension eye drops is basically the same as that of Example 1, except that in step S3, the homogenization conditions are: 9 times of homogenization at 50° C. and 1500 bar.
Embodiment 5
[0045] The type and consumption of the raw materials of the difluprednate suspension eye drops provided in this embodiment are exactly the same as those in Example 1. The preparation method of the suspension eye drops is basically the same as that of Example 1, except that in step S3, the homogenization conditions are: 65°C, 1000 bar for 6 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com