Transcription regulatory protein msir mutant protein of Rhizobium tianshanensis and its application in canavanine biosensor
A technique for mutating proteins and transcriptional regulation, applied in the field of bioengineering, can solve undiscovered problems and achieve the effect of improving regulatory activity and affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
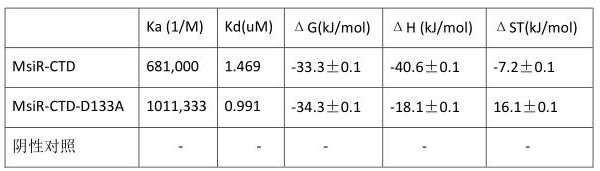
[0032] Embodiment 1: the preparation of transcription regulator protein MsiR (MsiR1-297aa) and MsiR-CTD (MsiR83-297aa) recombinant expression plasmid and recombinant expression transformant
[0033] (1) MsiR-CTD (MsiR83-297aa) is the C-terminal of the transcriptional regulatory protein MsiR. After the protein gene sequence of MsiR-CTD (MsiR83-297aa) is synthesized, the protein gene sequence is connected to the pET 21b empty vector (that is, the Carrier), at the same time with restriction endonucleases BamHI and NdeI double-digested overnight, then purified by agarose gel electrophoresis, DNA kit recovery, the recovered target fragment and the empty vector pET 21b were ligated in T4 DNA Under the action of the enzyme, connect at 4°C for 12 hours to obtain the recombinant plasmid pET21b-msiR-CTD, which is further transformed into competent cells BL21(DE3) to pick positive clones to obtain the recombinant expression transformant E.coli BL21(DE3) / pET21b-msiR-CTD.
[0034](2) Msi...
Embodiment 2
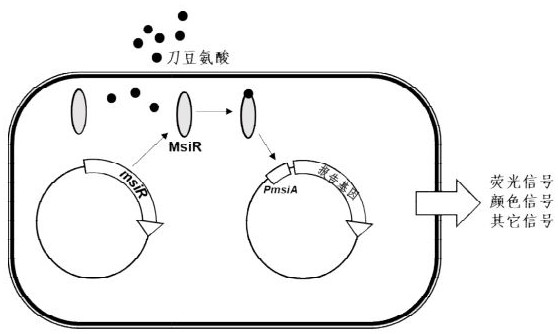
[0035] Example 2: Construction of a screening system for the activity of the transcriptional regulatory protein MsiR on canavanine
[0036] Using the msiR gene knockout strain of Bradyrhizobium in Tianshan TC4 as the starting strain, the PTCV141 plasmid and PTM5 plasmid were introduced into Tianshan Rhizobium (host cells) to construct a screening strain TC4 (PTCV141 plasmid and PTM5 plasmid), in which PTCV141 is a vector for constructing msiR gene mutation; PTM5 is to clone mCherry gene into the downstream of the promoter PmsiA regulated by MsiR protein, and it is used to quantitatively detect the influence of different MsiR mutant proteins on the transcriptional regulation of PmsiA and the expression of msiA promoter Expressed by the ratio of mCherry fluorescence value to OD600nm.
Embodiment 3
[0037] Embodiment 3: regulatory protein MsiR and MsiR-CTD mutant construction
[0038] The protein homology structure comparison was carried out through the NCBI database, and the 3FD3_A with the highest similarity in the protein structure database was selected as the template for homology modeling. The structural model of MsiR was built using DS Modeling2.5, and the 10.0 Å in the substrate binding pocket was selected. Amino acid, then carry out molecular docking with L-canavanine small molecule, and screen out 10 mutant amino acids that affect substrate binding through the conservation of amino acid sequence and docking scoring, and perform alanine mutation scanning. The primer sequences were designed for the mutated amino acids. The primer sequences are shown in Table 1. Using pET21b-msiR-CTD and PTCV141 as templates, one-step PCR was used, and high-fidelity polymerase was used for PCR. The PCR reaction conditions were as follows: PCR reaction system with a total volume of 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com