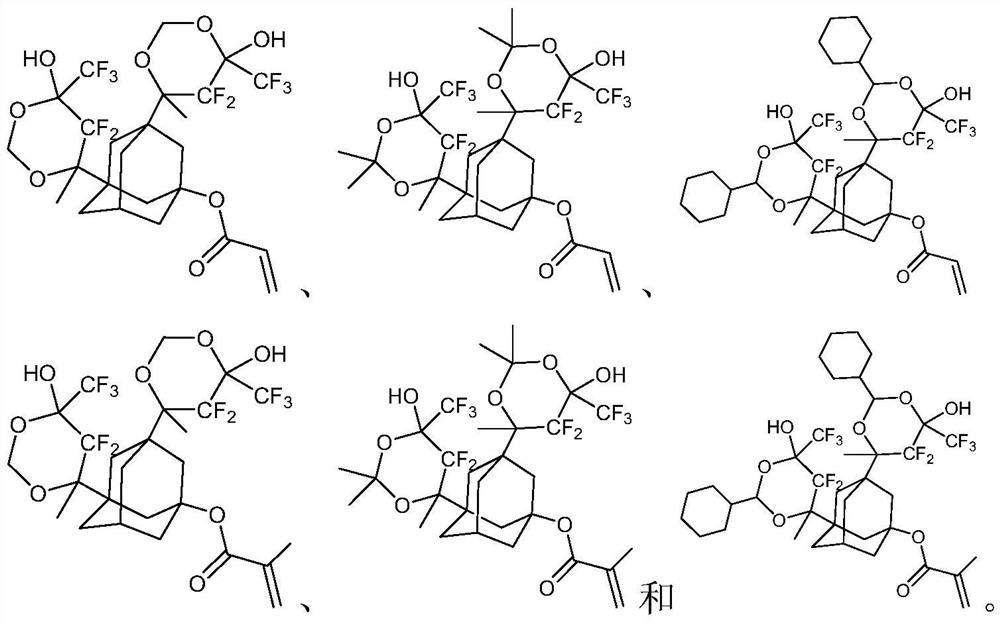
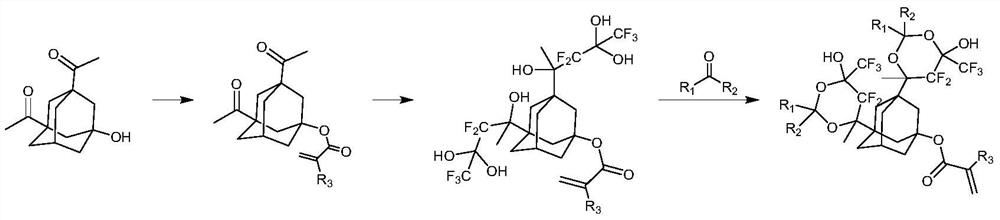
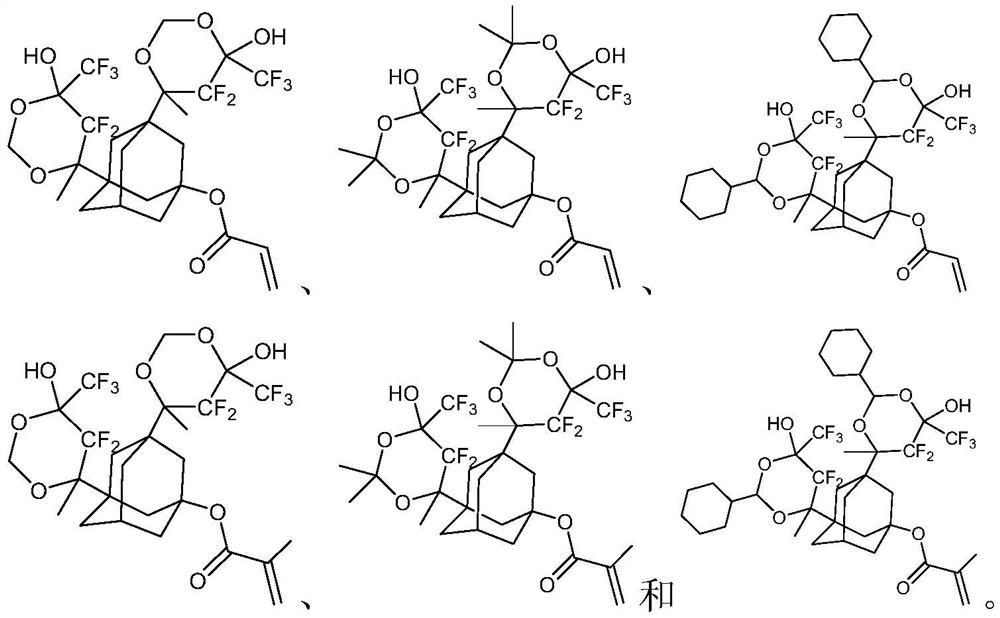
Photoresist resin monomer with adamantane structure and synthesis method thereof
A technology of resin monomer and synthesis method, applied in the field of photoresist resin monomer of adamantane structure and its synthesis, can solve the problem of poor T-top pattern, insufficient hydrophobicity of photoresist, affecting the resolution of photoresist, etc. problem, to achieve the effect of excellent etching resistance, excellent anti-swelling performance, and improved resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] The first step: 1,3-diacetyl-5-adamantanol (5g, 21mmol) was dissolved in tetrahydrofuran (50mL), triethylamine (6.4g, 63mmol) was added, cooled to 0 degrees Celsius with ice water, Under the protection of nitrogen, a solution of acryloyl chloride (2g, 22mmol) in tetrahydrofuran (20mL) was slowly added dropwise thereto, the reaction solution rose to room temperature and continued to react for 5 hours, the reaction solution was concentrated under vacuum to remove the solvent, and ethyl acetate (50mL) was added , adding saturated aqueous sodium bicarbonate (10mL), separating the organic phase, extracting the aqueous phase three times with ethyl acetate (50mL×3), combining the organic phases, washing the organic phase with saturated brine, drying over anhydrous sodium sulfate, and concentrating to obtain the compound 1-2 (5.5 g, 19 mmol, yield: 89.5%).
[0028] The second step: 1,1,1,3,3,3-hexafluoro-2-isopropanol (5.7g, 38mmol) was added to anhydrous tetrahydro...
Embodiment 2
[0031]
[0032] The raw material is the intermediate compound 1-3 of Example 1, compound 1-3 (10g, 16mmol), ion exchange resin (CAS: 39389-20-3) (1g), acetone (4.7g, 81mmol) are added to toluene (120mL), stirred at 60°C for 12 hours, filtered to remove the ion exchange resin, concentrated and purified by column chromatography to obtain compound P2 (5.1g, 7.5mmol, yield: 47.1%).
Embodiment 3
[0034]
[0035] The raw material is the intermediate compound 1-3 of Example 1, compound 1-3 (10g, 16mmol), ion exchange resin (CAS: 39389-20-3) (1g), 1-cyclohexylacetaldehyde (9g, 80mmol ) was added to toluene (150mL), stirred and reacted at 60 degrees Celsius for 12 hours, filtered to remove the ion exchange resin, concentrated and purified by column chromatography to obtain compound P3 (5.3g, 6.5mmol, 40.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com