Preparation method of 1, 1-dialkoxy alkane
A technology of alkyl and methyl, applied in the field of preparation of 1,1-dialkoxyalkane, can solve the problem of low yield of acetal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] According to the present invention, the preparation method of the 1,1-dialkoxyalkane comprises: in the presence of a catalyst, R 1 -OH Alcohol and R 2 For the contact reaction of aldehydes represented by CHO, the catalyst is a molecular sieve with a hydrogen type EWT structure.
[0016] According to the invention, in the presence of a catalyst, R 1 -OH Alcohol and R 2 The aldehyde represented by -CHO undergoes a condensation reaction to obtain R 2 -CH-(OR 1 ) 2 Acetals, that is, 1,1-dialkoxyalkanes.
[0017] According to the present invention, R 1 The alcohol shown in -OH can be a monohydric alcohol conventionally used in the art, for example, R 1 It may be a C1-C5 alkyl group, specifically, a methyl group, an ethyl group, a propyl group, a butyl group or a pentyl group. Alcohol R 1 Specific examples of -OH include, but are not limited to, methanol, ethanol, propanol, butanol, or pentanol.
[0018] According to the present invention, R 2 R in the aldehyde rep...
Embodiment approach
[0024] According to a specific embodiment of the present invention, the preparation method of the catalyst comprises: performing ammonium exchange on the raw EWT molecular sieve powder in an ammonium salt aqueous solution at 80-90°C, drying and calcining at 500-600°C for 1-5 hours ; Wherein, the ammonium salt is selected from one or more of ammonium nitrate, ammonium chloride and ammonium sulfate.
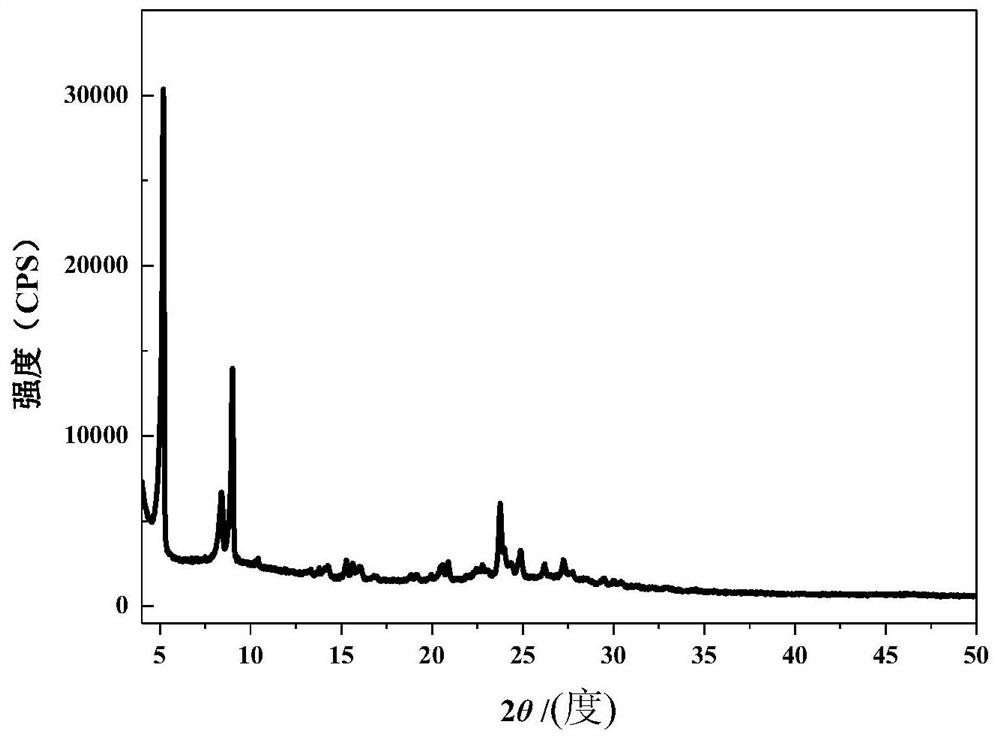
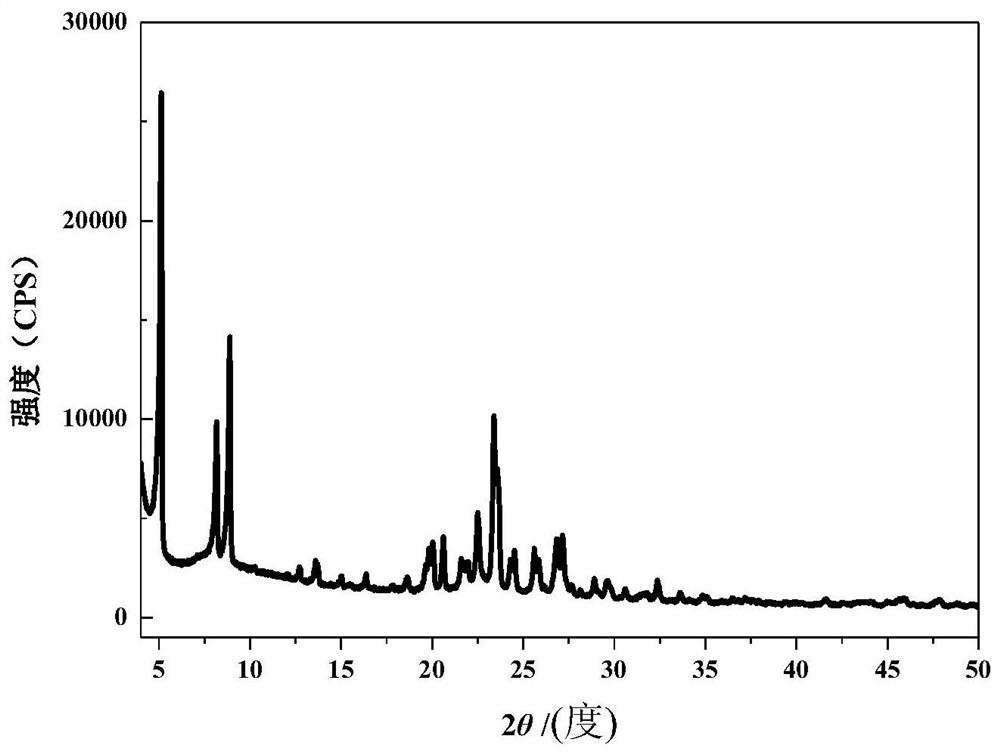
[0025]According to the present invention, the raw powder of molecular sieve with EWT structure can be obtained commercially, or can be prepared according to conventional methods in this field, such as the method disclosed in CN109422627A.
[0026] According to the present invention, the R 1 -OH Alcohol and R 2 The conditions of the aldehyde contact reaction shown in -CHO generally include reaction temperature and reaction pressure, wherein, the reaction temperature will ensure that R 1 -OH Alcohol and R 2 -CHO can undergo a condensation reaction under the catalysis of the cataly...
preparation example 1
[0044] Add 23.35g template agent R (1,5-bis(N-propylpyrrolidinium) pentane dihydroxide) aqueous solution (the content of R is 25% by mass) into a 45mL polytetrafluoroethylene container, add 1.25g sodium metaaluminate and 7.67g deionized water, stirred for 30 minutes until uniform, then added 9.64g solid silica gel (SiO 2 content is 93.37% by mass), stirred for 5 minutes and fully mixed, wherein the molar ratio of each component is: SiO 2 / Al 2 o 3 =90,H 2 O / SiO 2 =9.82, template R / SiO 2 = 0.15, OH - / SiO 2 = 0.08.
[0045] The above mixture was put into a 45mL polytetrafluoroethylene-lined steel autoclave, covered and sealed, and the autoclave was placed in a rotating convection oven with the rotation speed set at 20rpm, and reacted at 150°C for 5 days. Take out the autoclave and let it cool down to room temperature quickly, separate the mixture on a 5000rpm high-speed centrifuge, collect the solid, wash it thoroughly with deionized water, and dry it at 100°C for 5 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com