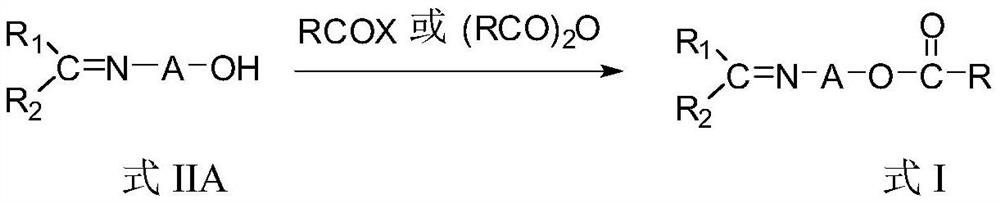
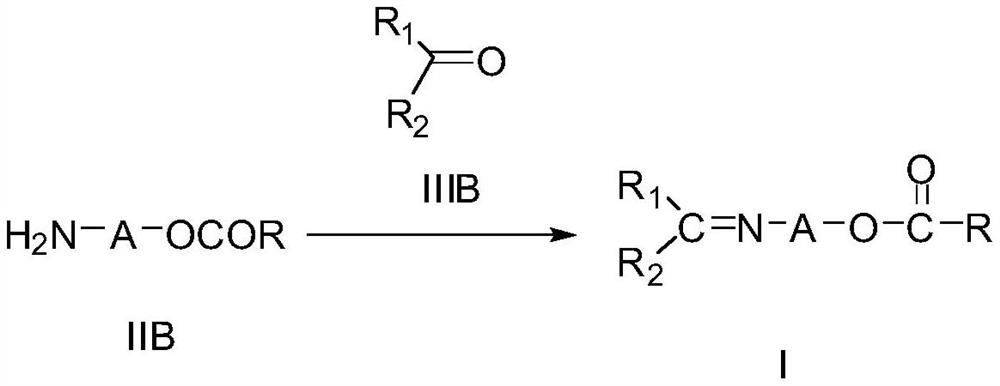
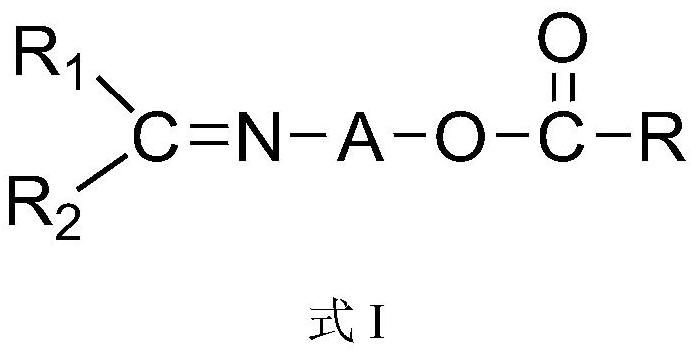Novel imine compound and preparation method thereof
A compound and imine technology, which is applied in the field of new imine ester compounds and their preparation, can solve the problem of unremarkable catalyst effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0028] According to some embodiments of the present invention, the substituent is selected from halogen, hydroxyl, amino, C 6 -C 20 Aryl, C 7 -C 20 Aralkyl, C 7 -C 20 Alkaryl, C 1 -C 10 Alkyl and C 1 -C 20 Alkoxy, preferably selected from halogen, hydroxy, amino, phenyl, naphthyl, benzyl, phenethyl, C 1 -C 6 Alkyl-substituted phenyl, C 1 -C 10 Alkyl and C 1 -C 20 Alkoxy, more preferably selected from fluorine, chlorine, bromine, iodine, hydroxyl, amino, phenyl, naphthyl, benzyl, phenethyl, methyl, ethyl, isopropyl, n-propyl, n-butyl base, isobutyl, tert-butyl, n-pentyl, isopentyl, n-hexyl, methoxy, ethoxy, isopropoxy, n-propoxy, n-butoxy, isobutoxy, tert One or more of butoxy, n-pentyloxy, isopentyloxy, and n-hexyloxy.
[0029] According to some embodiments of the present invention, R 1 and R 2 each independently selected from hydrogen, C with or without substituents 1 -C 20 Alkyl, C with or without substituents 2 -C 20 Alkenyl, C with or without substitue...
Embodiment 1
[0071] The synthesis of embodiment 1 compound benzoic acid (3-benzylidene amino propyl ester)
[0072]
[0073] Method 1: In a 250 ml three-necked flask, add 3.26 g of 3-(benzylidene amino) propanol, 120 ml of THF and 2.15 ml of triethylamine after purging with nitrogen, and add 2.80 g of benzene dropwise at room temperature Formyl chloride, stir well. Stir the reaction for 4 hours, then raise the temperature and reflux for 8 hours. After concentrating under reduced pressure, recrystallize from a mixed solution of diethyl ether / petroleum ether (1:50) to obtain pale yellow crystals, and dry in vacuo to obtain 2.47 g of the product (yield 40%). 1 H-NMR (δ, ppm, TMS, CDCl 3 ):8.13-8.10(1H,m,=CH),7.97-7.94(2H,m,ArH),7.63-7.61(2H,m,ArH),7.46-7.44(1H,m,ArH),7.36-7.34 (2H,m,ArH),7.30-7.28(3H,m,ArH),4.25-4.23(2H,t,OCH 2 ),3.55-3.53(2H,m,=NCH 2 ),2.12-2.10(2H,t,CH 2 ).
[0074] Method 2: In a 250 ml three-necked flask, add 3.58 g of (3-amino)propyl benzoate, 120 ml of isoprop...
Embodiment 2
[0075] The synthesis of embodiment 2 compound benzoic acid [4-(3,5-di-tert-butylbenzylidene amino)-2-pentyl ester]
[0076]
[0077] In a 250 ml three-neck flask, add 3.03 g of 4-(3,5-di-tert-butylbenzylidene amino)-2-pentanol, 120 ml of THF and 1.10 ml of triethylamine after purging with nitrogen 1.40 g of benzoyl chloride was added dropwise at room temperature and stirred evenly. After stirring for 4 hours, the temperature was raised to reflux for 10 hours. After concentrating under reduced pressure, recrystallize from a mixed solution of diethyl ether / petroleum ether (1:50) to obtain light yellow crystals, and dry in vacuo to obtain 2.10 g of the product (yield 51%). 1 H-NMR (δ, ppm, TMS, CDCl 3 ):8.23-8.22(1H,m,=CH),7.99-7.98(2H,m,ArH),7.46-7.44(3H,m,ArH),7.36-7.34(3H,m,ArH),4.20-4.18 (1H,t,OCH),3.35-3.32(1H,t,NCH),2.04-2.03(2H,m,CH 2 ), 1.41-1.39 (3H,d,CH 3 ),1.35-1.32(18H,m,CH 3 ),1.28-1.26(3H,d,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com