Novel synthesis method of 4-methylthiazole-5-formaldehyde
A technique for the synthesis of methylthiazole, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., and can solve the problem of low reaction yield and low yield , harsh conditions and other issues, to achieve the effect of clean reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
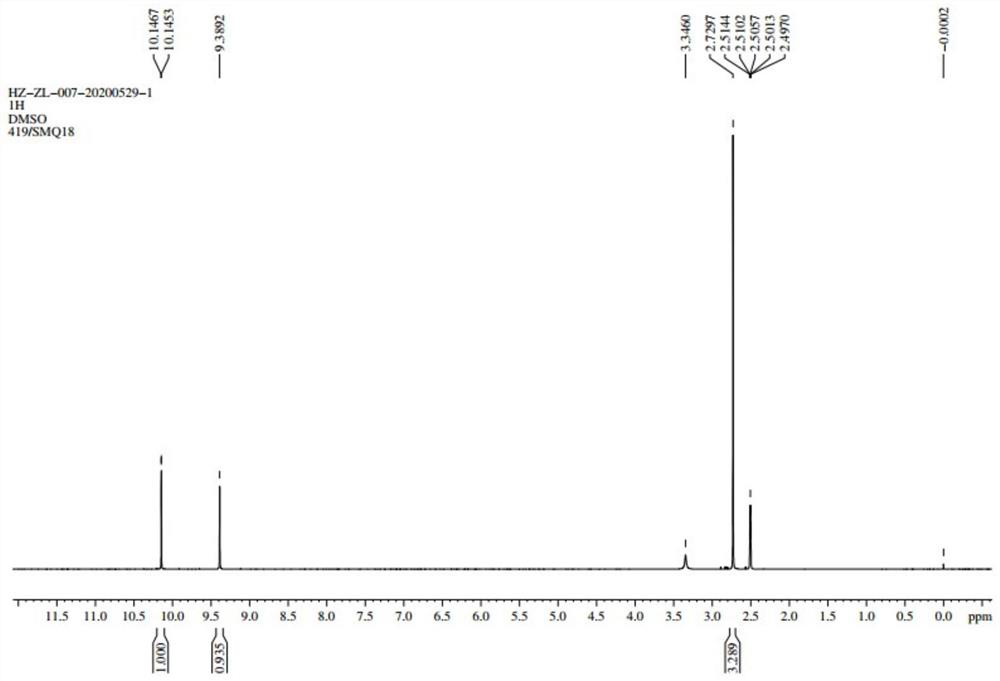
[0023] Add 129.2g (1.0mol) of 4-methylthiazole-5-methanol, 3.44g (0.02mol) of 4-hydroxy-2,2,6,6-tetramethylpiperidine oxide, trichloro 8.1 g (0.05 mol) of iron oxide, 3.5 g (0.05 mol) of sodium nitrite and 400 mL of 1,2-dichloroethane were stirred and reacted in air for 8 hours at room temperature and normal pressure.
[0024] Sampling and detection to the end of the reaction; transfer the reaction solution to a separatory funnel, wash twice with saturated sodium thiosulfate solution, dry the organic phase with anhydrous sodium sulfate after liquid separation, and recover the solvent by distillation under reduced pressure to obtain a light yellow The solid was recrystallized with n-heptane to obtain 4-methylthiazole-5-carbaldehyde in the form of light yellow crystal powder with a content of 99% and a yield of 83%.
Embodiment 2
[0026] 129.2g (1mol) of 4-methylthiazole-5-methanol, 5.4g (0.03mol) of 2,2,6,6-tetramethylpiperidine oxide, 12g (0.1mol) of 30% hydrochloric acid, and sodium nitrite Put 3.5g (0.05mol) into a 1L three-necked flask, add 400mL of dichloromethane, stir evenly, and introduce air, and react at room temperature for 10 hours.
[0027] After the reaction was completed, the reaction solution was transferred to a separatory funnel, washed twice with saturated sodium bicarbonate solution, and the organic phase was dried overnight with anhydrous sodium sulfate after liquid separation, and the solvent was recovered by distillation under reduced pressure to obtain a light yellow solid, and then Heptane was recrystallized to obtain light yellow crystal powder 4-methylthiazole-5-carbaldehyde with a content of 99% and a yield of 96%.
Embodiment 3
[0029] 129.2g (1mol) of 4-methylthiazole-5-methanol, 18.6g (0.1mol) of 4-methoxy-2,2,6,6-tetramethylpiperidine oxide, and 11.9g of potassium bromide ( 0.1mol) and 10.3g (0.1mol) of tert-butyl nitrite were put into a 1L autoclave, 400mL of dichloromethane was added, after stirring evenly, oxygen was introduced, the temperature was raised to 80°C, and the reaction was refluxed for 6 hours.
[0030] After the reaction is completed, cool to room temperature, carefully remove the pressure, transfer the reaction solution to a separatory funnel, dry the organic phase with anhydrous sodium sulfate overnight, and recover the solvent by distillation under reduced pressure to obtain a light yellow solid, and then use n-heptyl Alkane recrystallized to obtain light yellow crystalline powder 4-methylthiazole-5-carbaldehyde with a content of 99% and a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com