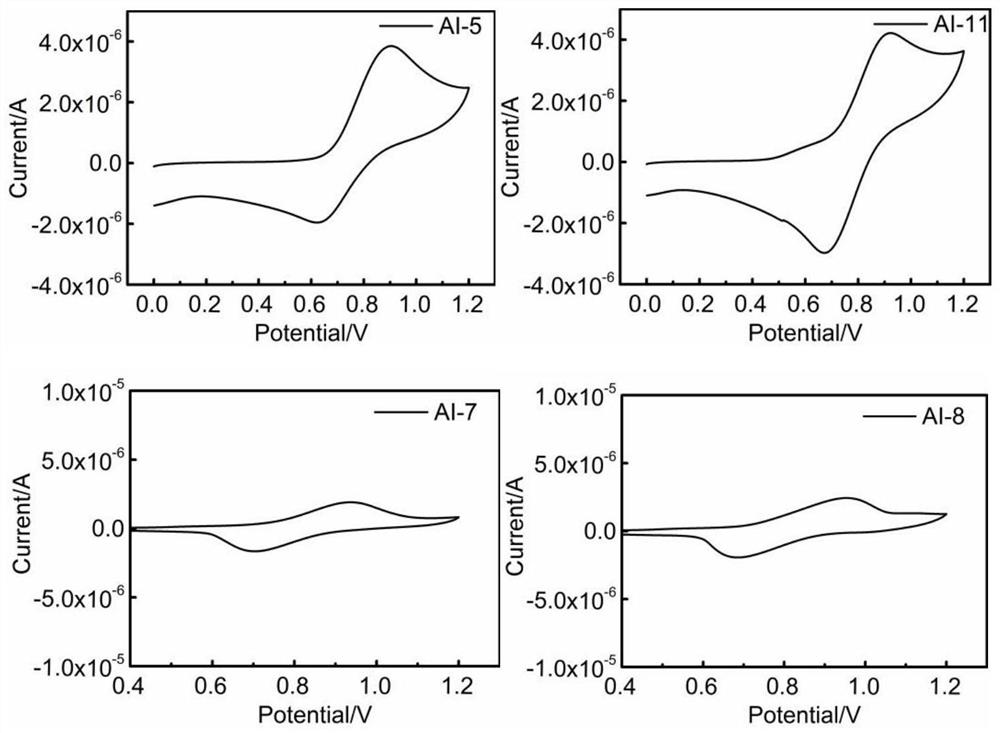
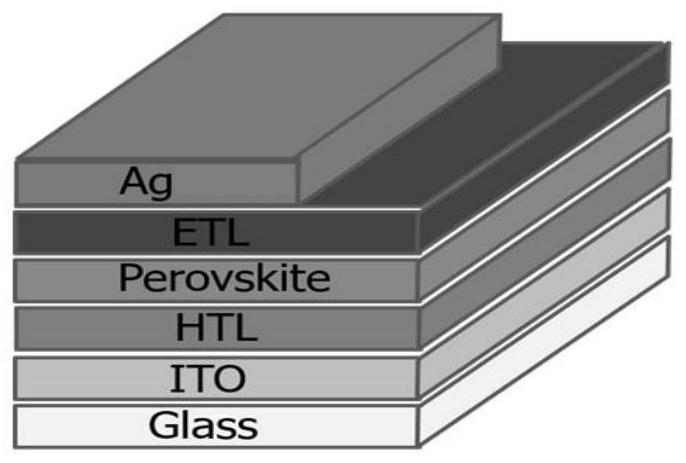
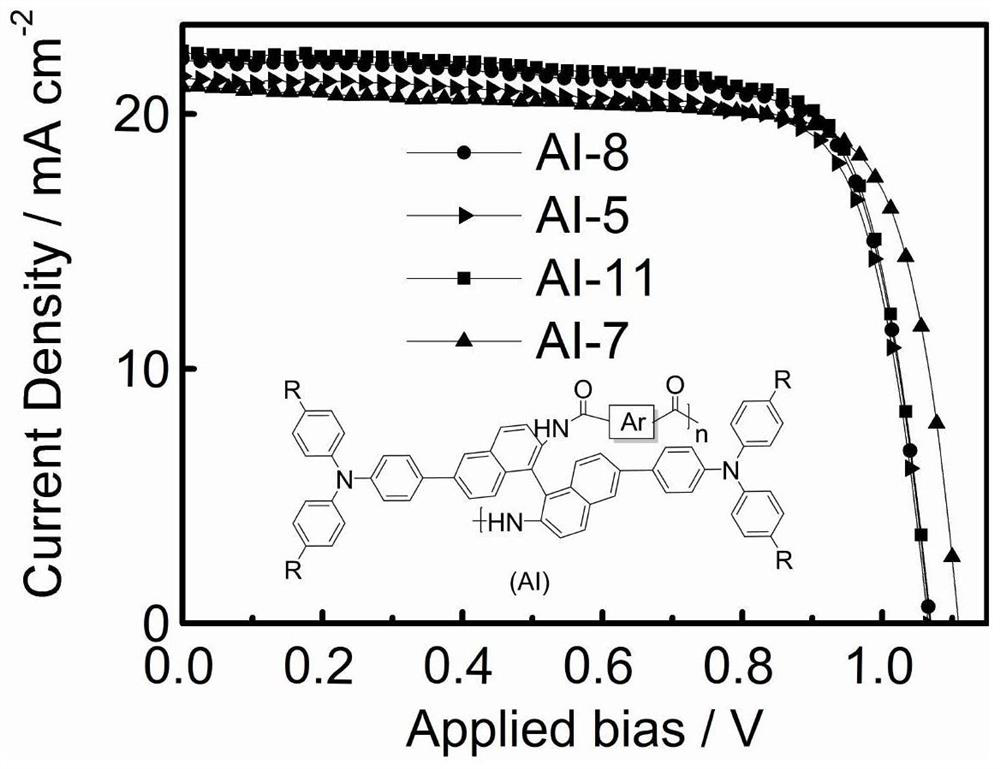
Amide bridged organic polymer hole transport material and synthesis method and application thereof
A technology for hole transport materials and polymers, which is applied in the field of amide bridged organic polymer hole transport materials and their synthesis, and can solve the problems of complex synthesis, high cost, and poor photoelectric conversion efficiency of batteries.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1, Preparation of 6,6'-bis(4-(bis(4-(4-methylthiophenyl)amino)phenyl)-2,2'-binaphthylamine Z11:
[0039]
[0040] Dibromobinaphthylamine (1.144g, 2.6mmol) and 4-boronic acid pinacol ester-4', 4'-dimethylthiotriphenylamine (2.8015g, 6.5mmol), added tetrakis (triphenyl Phosphine) palladium (0.601g, 0.52mmol), adding sodium carbonate aqueous solution (2.2048g, 20.8mmol, 20ml) was replaced by nitrogen, condensed and refluxed at 100°C for 3h, the reaction solution was cooled to room temperature, extracted, column chromatography ( Petroleum ether (PE) / ethyl acetate (EA)=2:1, vol%) was purified to obtain 1.15 g of polymer monomers with a yield of 46.37%.
[0041] 1H NMR (400MHz, DMSO) δ8.07 (d, J = 1.1Hz, 2H), 7.89 (d, J = 8.9Hz, 2H), 7.66 (d, J = 8.6Hz, 4H), 7.48 (d, J =8.8Hz, 2H), 7.33(s, 2H), 7.27(d, J=8.7Hz, 8H), 7.06(dd, J=14.2, 8.6Hz, 12H), 6.94(d, J=8.8Hz, 2H ),4.81(s,4H),2.50(s,12H).
[0042] 2. Preparation of polymer
Embodiment 2
[0043] Embodiment 2, the preparation of polymer AI-1
[0044]
[0045] Add terephthalic acid (1.328g, 8.0mmol) into the reaction flask, add excess dry toluene, 1-2 drops of dry N,N-dimethylformamide, inject excess thionyl chloride with a syringe, reflux and stir overnight, spin Dry to obtain pure terephthaloyl chloride. 6,6'-bis(4-(bis(4-(4-methoxyphenyl)amino)phenyl)-2,2'-binaphthylamine (1.4 g, 1.57 mmol) was dissolved in excess tetrahydrofuran , inject triethylamine (0.4757g, 1.57mmol) with a syringe, add the terephthaloyl chloride (0.3187g, 1.57mmol) that makes, react 3 days under room temperature. Spin dry reaction solution, add clean rotor, use benign The solvent was dissolved, and then a poor solvent was added to precipitate a solid, filtered with suction, and the filter cake was taken and dried to obtain 1.3 g of the target polymer, with a yield of 76%.
Embodiment 3
[0046] Embodiment 3, the preparation of polymer AI-2
[0047]
[0048] Add 2,3,5,6-tetrafluoroterephthalic acid (1.9g, 8.0mmol) into the reaction flask, add excess dry toluene, 1-2 drops of dry N,N-dimethylformamide, inject excess Thionyl chloride was stirred under reflux overnight, and spin-dried to obtain pure 2,3,5,6-tetrafluoroterephthaloyl chloride. 6,6'-bis(4-(bis(4-(4-methoxyphenyl)amino)phenyl)-2,2'-binaphthylamine (1.4 g, 1.57 mmol) was dissolved in excess tetrahydrofuran , injected triethylamine (0.4757g, 1.57mmol) with a syringe, added the prepared 2,3,5,6-tetrafluoroterephthaloyl chloride (0.4308g, 1.57mmol), and reacted for 3 days at room temperature. Dry the reaction solution, add a clean rotor, dissolve with a good solvent, then add a poor solvent to precipitate a solid, filter with suction, take the filter cake and dry to obtain 1.5 g of the target polymer, with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com