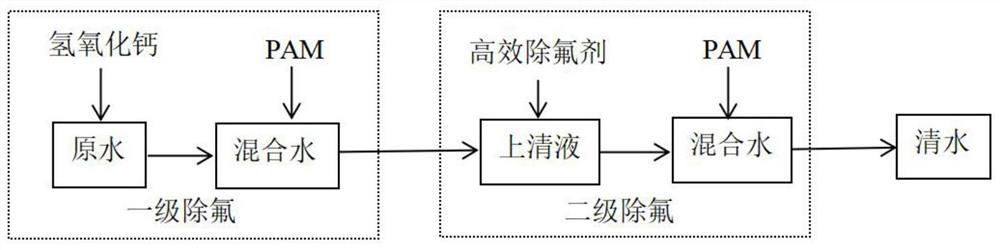
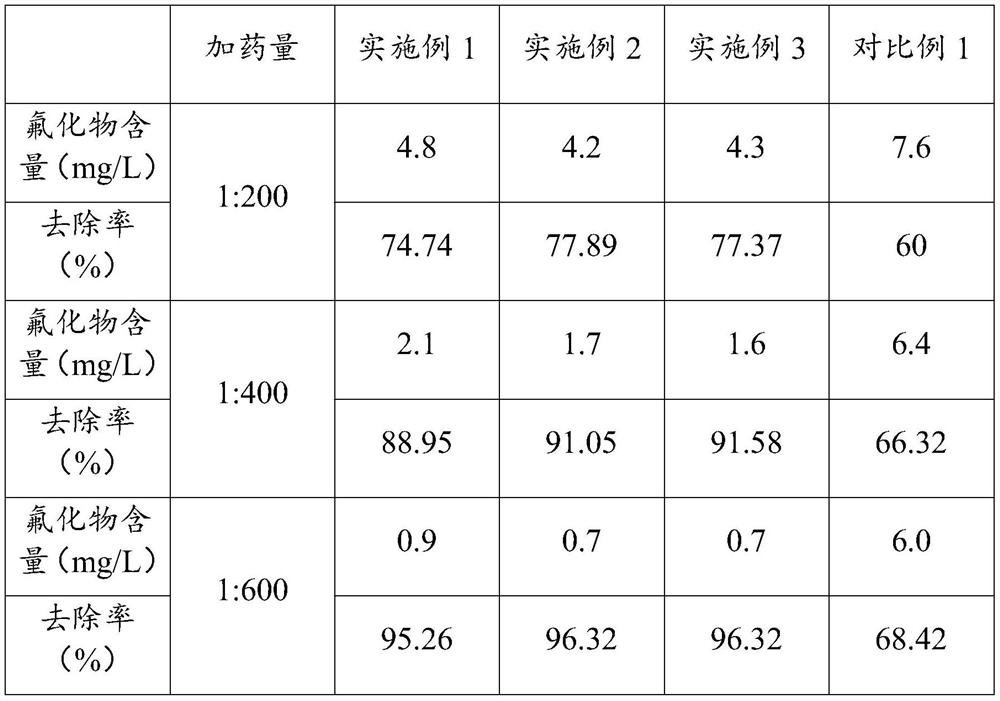
Deep defluorination agent and preparation method thereof
A chemical and advanced technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of low adsorption capacity of adsorbents, low water content of sedimentary sludge, and high fluoride content in wastewater. The effect of good dehydration performance, fast mass density precipitation and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Iron salt is nano iron silicate, and its preparation method comprises the following steps:
[0038] At room temperature, after mixing ferrous sulfate monohydrate and sodium silicate evenly, add a mixed solution of nitric acid with a mass concentration of 98% and sulfuric acid with a mass concentration of 93% and stir to react, allowing the raw materials to react at 0-90°C After 24-48 hours, after the reaction is complete, the product is taken out and pulverized, and the product is left for 24-48 hours to obtain nanometer iron silicate.
[0039] In order to further optimize the above technical scheme, the acid includes one or more of hydrochloric acid, sulfuric acid and phosphoric acid.
[0040] Further, the calcium salt includes one or more of calcium chloride, calcium citrate and calcium acetate.
[0041] Further, aluminum salts include polyaluminum chloride.
[0042] Further, the magnesium salt includes one or more of magnesium chloride, magnesium sulfate, magnesium...
Embodiment 1
[0053] A novel defluoridation agent, the preparation method comprising the following steps:
[0054] S1: Preparation of nano iron silicate: at room temperature, mix and stir the solid raw material ferrous sulfate monohydrate and sodium silicate in a molar ratio of 1:0.017, then add nitric acid with a mass concentration of 98% and a mass concentration of 93% A mixed solution of sulfuric acid, wherein the molar ratio of ferrous sulfate monohydrate: nitric acid: sulfuric acid is 1:0.330:1.349, the raw materials are stirred and reacted, and the heat released during the reaction will cause the reaction temperature to rise continuously and accompanied by the formation of NO , to accelerate the reaction, the reaction time is 24 hours, and the reaction temperature is controlled at 0-90°C to complete the oxidation and polymerization reactions between the raw materials. After the reaction is complete, the reactants are taken out and crushed, and placed for 24-48 hours to obtain nano Iro...
Embodiment 2
[0060] A novel defluoridation agent, the preparation method comprising the following steps:
[0061] S1: Preparation of nano-ferric silicate: at room temperature, mix and stir the solid raw material ferrous sulfate monohydrate and sodium silicate in a molar ratio of 1:0.062, then add nitric acid with a mass concentration of 98% and a mass concentration of 93% A mixed solution of sulfuric acid, wherein the molar ratio of ferrous sulfate monohydrate: nitric acid: sulfuric acid is 1:0.371:1.413, the raw materials are stirred and reacted, and the heat released during the reaction will cause the reaction temperature to rise continuously and accompanied by the formation of NO , to accelerate the reaction, the reaction time is 35 hours, and the reaction temperature is 0-90°C, so as to complete the oxidation and polymerization reactions between the raw materials. After the reaction is complete, the reactants are taken out and crushed, and placed for 24-48 hours to obtain nano-silicon ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com