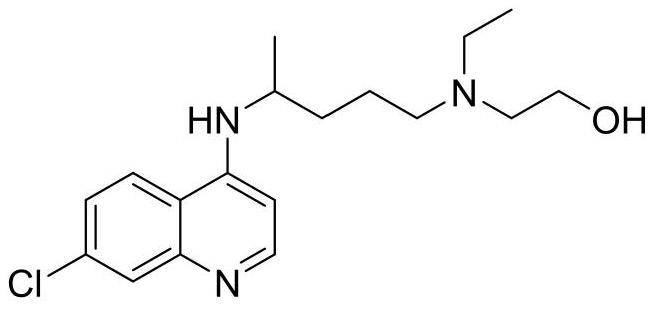
Synthesis method of hydroxychloroquine sulfate
A technology of hydroxychloroquine sulfate and sulfuric acid, applied in the direction of organic chemistry, can solve the problems of cumbersome operation, unfriendly environment, long reaction time, etc., and achieve the effect of simple overall process, strong operability and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
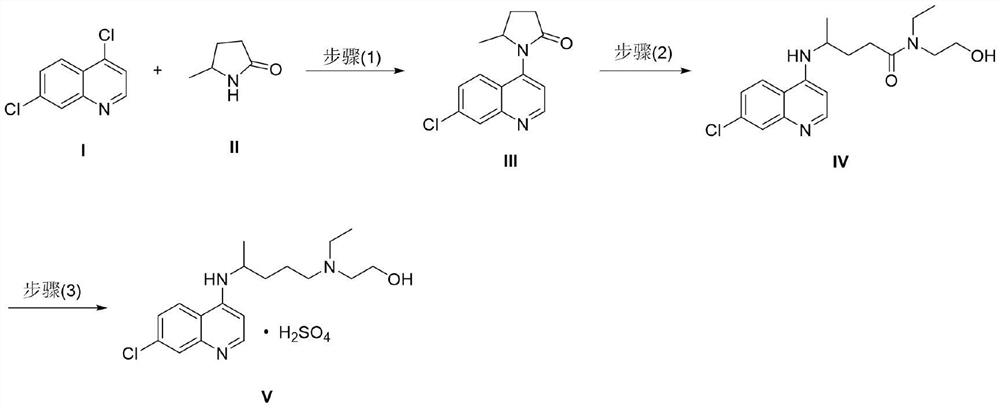
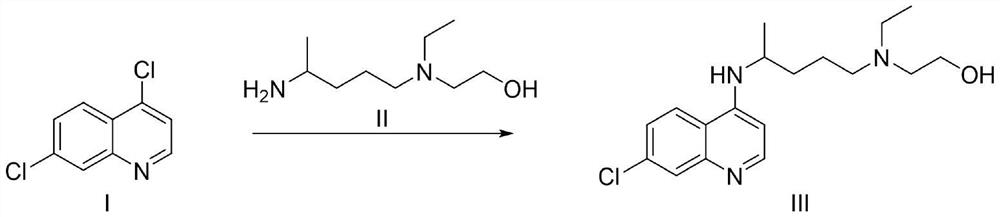
[0041] The preparation of embodiment 1 formula III compound
[0042]
[0043] At room temperature, the formula 4,7-dichloroquinoline (19.8g, 100.0mmol), 5-methyl-2-pyrrolidone (14.9g, 150.0mmol), 4,5-bisdiphenylphosphine-9,9 -Dimethylxanthene (578mg, 1.0mmol), palladium acetate (224 mg, 1.0mmol) and cesium carbonate (48.8g, 150.0mmol) were mixed in 1,4-dioxane (150ml), nitrogen replacement Three times, under the protection of nitrogen, the temperature was raised to reflux and stirred. It was detected by TLC that the raw materials were consumed completely, the reaction was stopped, and the temperature was cooled to room temperature. Add ethyl acetate (300ml) and water (300ml) into the system, stir for a while, separate the layers, extract the aqueous phase with ethyl acetate (150ml) again, and combine the organic phases. The organic phase was washed successively with water (200ml) and saturated sodium chloride (200ml), and concentrated to dryness under reduced pressure to ...
Embodiment 4
[0048] The preparation of embodiment 4 formula IV compound
[0049]
[0050] At room temperature, the compound of formula III (2.6g, 10.0mmol) and N-ethylethanolamine (4.5g, 50.0mmol) were mixed, heated to 80°C and stirred. It was detected by TLC that the raw materials were completely consumed, the reaction was stopped, and the temperature was cooled to room temperature to obtain an orange mucus. Recrystallized from isopropanol (4ml) and n-hexane (10ml) to obtain 2.2g of a yellow solid with a yield of 63%, which is the compound of formula IV.
Embodiment 5
[0051] The preparation of embodiment 5 formula IV compound
[0052] At room temperature, aluminum trichloride (1.7g, 13.0mmol) was mixed with dichloromethane (20ml), and the temperature was lowered to 0°C under nitrogen protection; the compound of formula III (2.6g, 10.0mmol) and N-ethyl Add ethanolamine (2.7 g, 25.0 mmol) into the above system, and keep stirring at an internal temperature of 0-5°C. TLC detects that the raw materials are consumed completely, quench the reaction with water (30 ml), adjust the system pH=7 to 8 with aqueous sodium hydroxide solution (1N), stir for a while, separate the layers, discard the aqueous layer, and concentrate the organic layer to dryness under reduced pressure. Get orange slime. Recrystallized from isopropanol (4ml) and n-hexane (10ml) to obtain 2.6g of a yellow solid with a yield of 74%, which is the compound of formula IV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com