Novel lithium ferric manganese phosphate positive electrode material synthesis method
A technology of lithium iron manganese phosphate and cathode material, applied in phosphorus compounds, chemical instruments and methods, battery electrodes, etc., can solve the problems of low discharge capacity ratio and poor cycle stability, and achieve uniform composition, stable cycle performance and high voltage. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
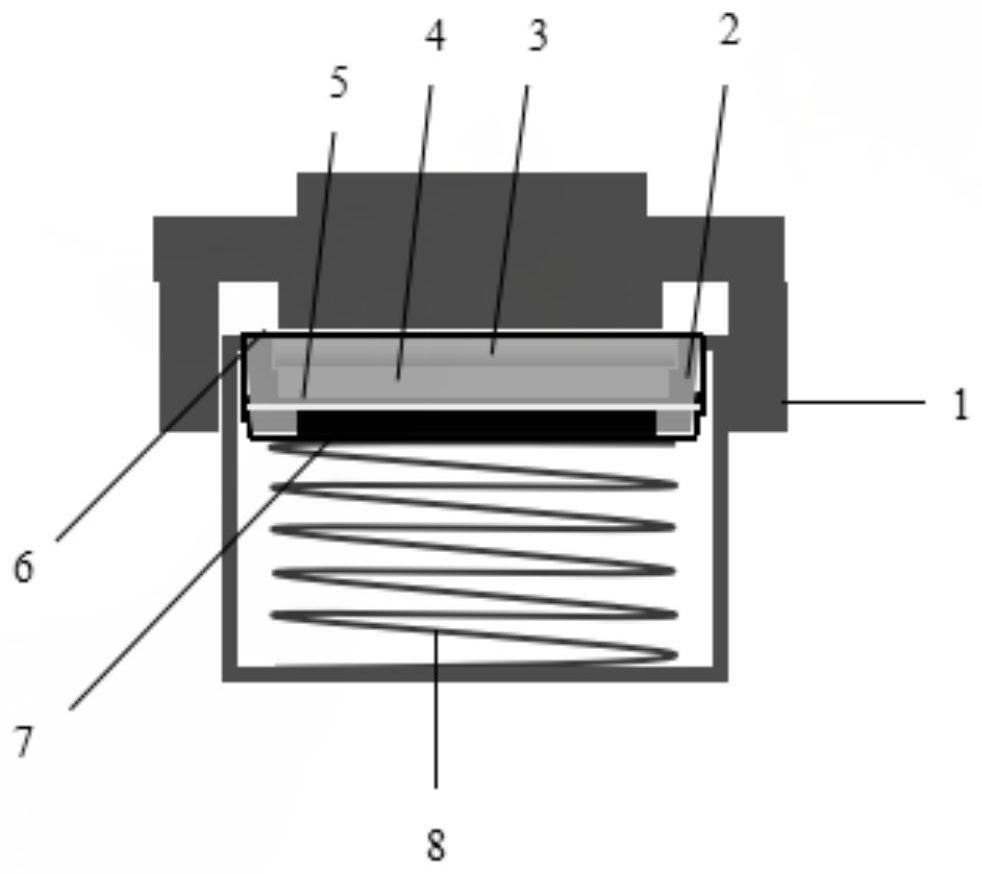
Image
Examples
Embodiment 1
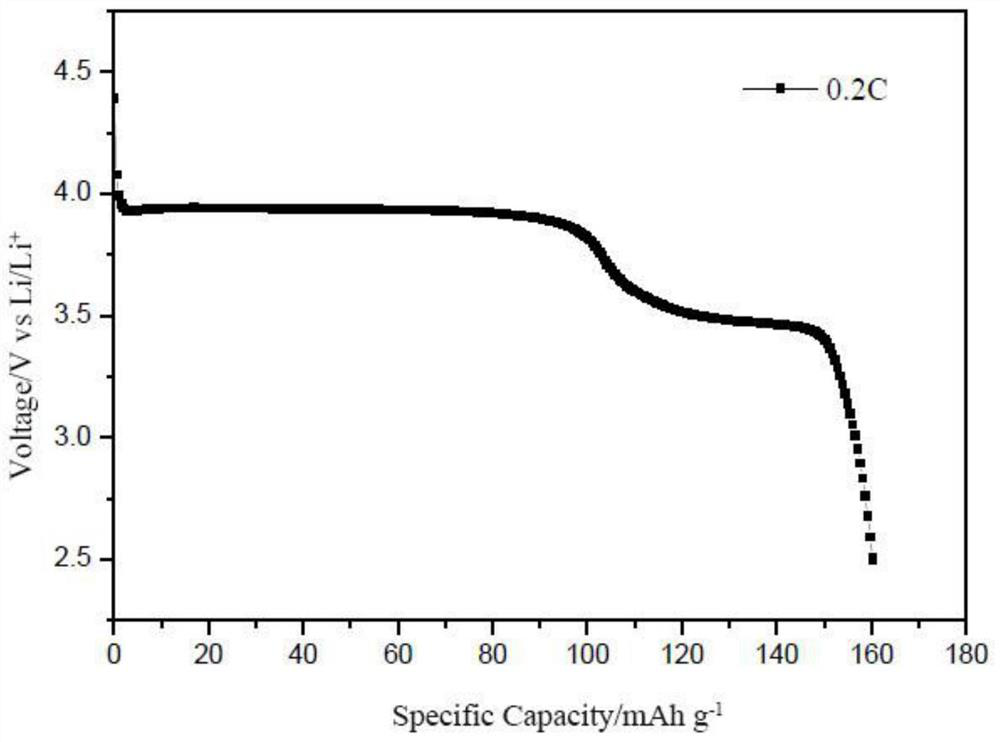
[0038] 60.9 g of analytically pure manganese dioxide, 68 ml of 85% phosphoric acid, and 302 ml of deionized water were placed in a container and continuously stirred for 4 hours, then 93 ml of aniline was added, and the stirring was continued for 2 hours to obtain polyaniline. Separately weigh 24 g of ferric oxide, 37 g of lithium carbonate, 22.3 g of glucose monohydrate, 1.1 g of hydroxyalkyl hydroxy dimerized linoleyl ether, 0.5 g of N-palmitoyl hydroxyproline cetyl ester, and 2.1 g of polyvinylpyrrolidone. g, added to the above polyaniline liquid, and ground and stirred for 12 hours. After drying in air atmosphere, calcining in air atmosphere, the calcination temperature is 800° C., and the calcination time is 10 h, to obtain the precursor.
[0039]Add 22.3g of glucose monohydrate and 100g of water to the precursor again as a grinding medium, put it into a high-speed ball mill and grind for 8 hours to obtain a grinding slurry with a particle size D50 of 0.85um.
[0040] Pu...
Embodiment 2
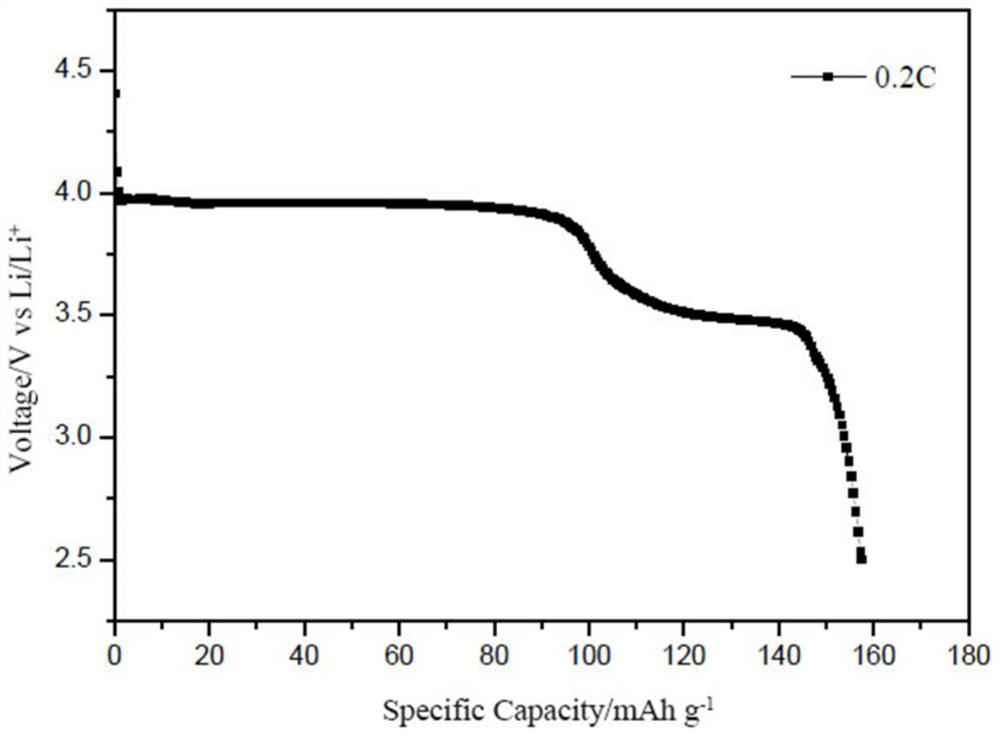
[0043] 60.9 g of analytically pure manganese dioxide, 68 ml of 85% phosphoric acid, and 302 ml of deionized water were placed in a container and continuously stirred for 3 hours, then 93 ml of aniline was added, and stirring was continued for 1 hour to obtain polyaniline. Separately weigh 24g of ferric oxide, 24g of lithium hydroxide, 22.3g of glucose monohydrate, 1.6g of hydroxyalkyl hydroxydimer linoleyl ether, 0.7g of N-palmitoyl hydroxyproline cetyl ester, polyvinylpyrrolidone 2.4 g was added to the above polyaniline liquid, and ground and stirred for 8 hours. Then, after being dried in an air atmosphere, it is calcined in an air atmosphere at a calcining temperature of 500° C. and a calcining time of 8 hours to obtain a precursor.
[0044] The precursor was added with 22.3g of glucose monohydrate and 100g of water as a grinding medium, and was put into a high-speed ball mill for grinding for 8 hours to obtain a grinding slurry with a particle size D50 of 0.85 μm.
[0045...
Embodiment 3
[0048] 60.98g of analytically pure manganese dioxide, 68ml of 85% phosphoric acid, and 302ml of deionized water were placed in the container and continuously stirred for 2h, then 93ml of aniline was added, and the stirring was continued for 1h to obtain polyaniline. Separately weigh 24g of ferric oxide, 24g of lithium hydroxide, 38.5g of sucrose, 1.4g of hydroxyalkyl hydroxydimer linoleyl ether, 0.7g of N-palmitoyl hydroxyproline cetyl ester, and 2.3g of polyvinylpyrrolidone , added to the polyaniline liquid, grinding and stirring for 10h. Then, after being dried in an air atmosphere, it is calcined in an air atmosphere at a calcination temperature of 600° C. and a calcination time of 6 hours to obtain a precursor.
[0049] Add 38.5g sucrose to the precursor again, and add 100g water as a grinding medium, put it into a high-speed ball mill and grind it for 8 hours to obtain a grinding slurry with a particle size D50 of 0.85um.
[0050] Put the grinding slurry into a rake vacu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com