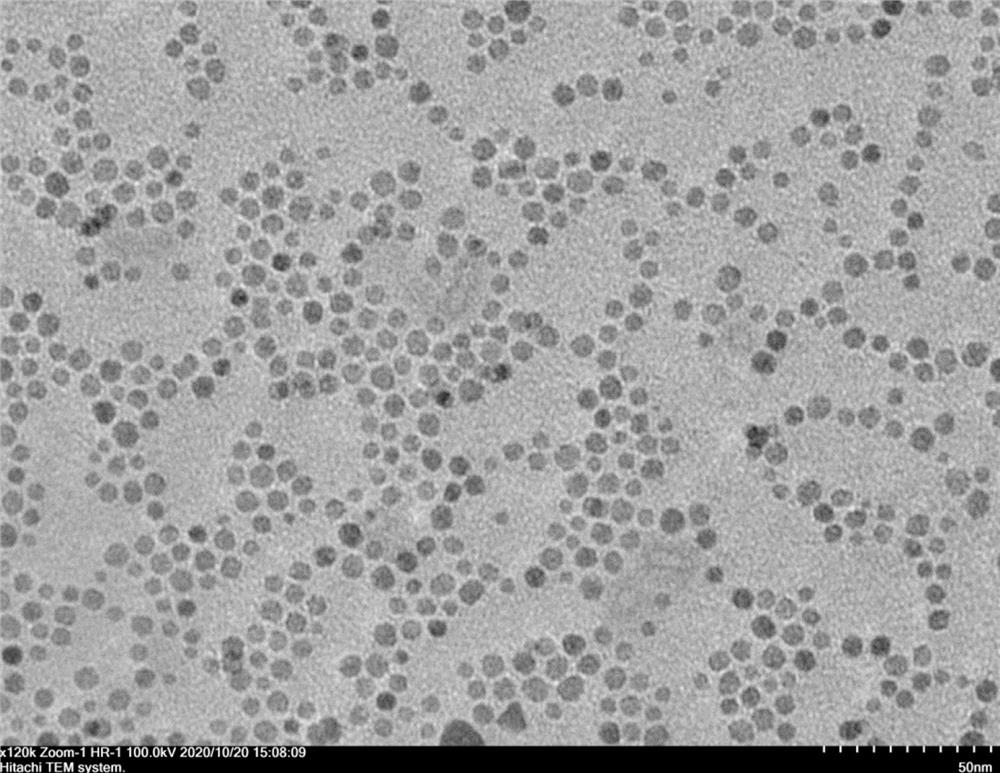
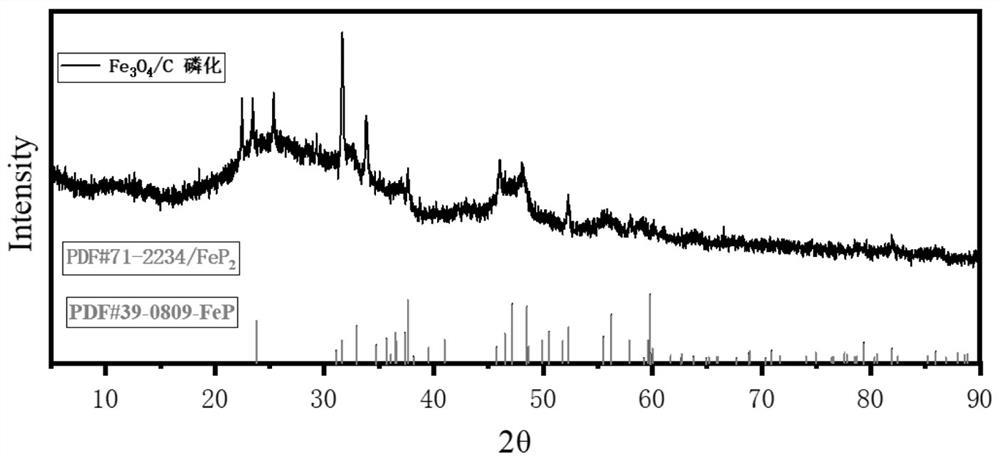
Preparation method of iron phosphide nano material and application of iron phosphide nano material as electrocatalyst
A technology of nanomaterials and iron phosphide, which is applied in the field of preparation of iron phosphide nanomaterials, can solve the problems of high price, difficulty in satisfying industrial production and application, and scarcity of platinum reserves, and achieve simple phosphating method and multiple surface active sites Accurate and controllable effects of points and experimental conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (01) Dissolve 0.7843g of ammonium ferrous sulfate hexahydrate in 15ml of ultrapure water and add dropwise to a uniform solution of 1.0g of sodium hydroxide dissolved in 5ml of ultrapure water, 10ml of oleic acid and 10ml of ethanol. After stirring well, a brown precipitate was produced.
[0040] (02) Put in a 100ml reactor, heat to 180°C, keep warm for 10 hours, cool the reactor to room temperature in an oven, and then take it out.
[0041] (03) Centrifuge and wash the generated ferric oxide with ethanol to obtain 0.1237 g of iron oxide particles, which are dispersed in 4 mL of cyclohexane for storage.
[0042] (04) Add 124 mg of carbon powder with a high specific surface area to the ferric oxide dispersed in cyclohexane obtained in step (03), and after ultrasonication, suction filtration, and drying, the ferric oxide loaded on the carbon powder is obtained. iron nanoparticles.
[0043] (05) Place the porcelain boat with 1g of sodium hypophosphite on the air inlet sid...
Embodiment 2
[0052] (01) Dissolve 0.54g ferric chloride hexahydrate in 15ml ultrapure water and add dropwise to a homogeneous solution of 1.0g sodium hydroxide dissolved in 5ml ultrapure water, 10ml oleic acid and 10ml ethanol. After stirring evenly, a brick red precipitate was produced.
[0053] (02) Put in a 100ml reactor, heat to 180°C, and continue heating for 10 hours, then cool the reactor to room temperature in an oven and take it out.
[0054] (03) The generated iron oxide particles were centrifuged three times with ethanol and washed to obtain 0.128 g of iron oxide, which were dispersed in 4 mL of cyclohexane for storage.
[0055] (04) Add 128 mg of carbon powder with high specific surface area to the cyclohexane obtained in step (03) with iron oxide particles dispersed therein, and after ultrasonication, suction filtration, and drying, iron oxide nanoparticles loaded on the carbon powder are obtained.
[0056] (05) Place the porcelain boat containing 1 g of sodium hypophosphite on...
Embodiment 3
[0066] (01) Dissolve 0.7843 g ammonium ferrous sulfate hexahydrate in 15 ml ultrapure water and add dropwise to a homogeneous solution of 0.5 g sodium hydroxide dissolved in 5 m water, 10 oleic acid and 10 ml ethanol. After stirring well, a light brown precipitate was produced.
[0067] (02) Put in a 100ml reactor, heat to 180°C, and continue heating for 10 hours, then cool the reactor to room temperature in an oven and take it out.
[0068] (03) The generated iron oxyhydroxide particles were centrifuged and washed with ethanol to obtain 0.144 g of iron oxide particles, which were dispersed in 4 mL of cyclohexane for storage.
[0069] (04) Add 144 mg of carbon powder with a high specific surface area to the cyclohexane obtained in step (03) dispersed with iron oxyhydroxide particles, and after ultrasonication, suction filtration, and drying, the ferric iron tetroxide loaded on the carbon powder is obtained nanoparticles.
[0070] (05) Put the carbon-loaded iron oxyhydroxide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Average length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com